writing chemical equations
... Active metals replace less active metals from their compounds in aqueous solution. Magnesium turnings are added to a solution of iron(III) chloride. Active metals replace hydrogen in water. Sodium is added to water. Active metals replace hydrogen in acids. Lithium is added to hydrochloric acid ...
... Active metals replace less active metals from their compounds in aqueous solution. Magnesium turnings are added to a solution of iron(III) chloride. Active metals replace hydrogen in water. Sodium is added to water. Active metals replace hydrogen in acids. Lithium is added to hydrochloric acid ...
Chemistry@YIA – additional information
... 0.429 g of crystalline sodium carbonate (Na2CO3.xH2O) required 15.0 cm3 of 0.2 mol/dm3 HCl for neutralisation. Calculate the Mr of Na2CO3.xH2O and x. Na2CO3.xH2O(s) + 2 HCl(aq) 2 NaCl(aq) + CO2(g) + (x+2) H2O(l) ...
... 0.429 g of crystalline sodium carbonate (Na2CO3.xH2O) required 15.0 cm3 of 0.2 mol/dm3 HCl for neutralisation. Calculate the Mr of Na2CO3.xH2O and x. Na2CO3.xH2O(s) + 2 HCl(aq) 2 NaCl(aq) + CO2(g) + (x+2) H2O(l) ...
Solutions, Acids, and Bases
... solute to a liquid solvent will usually raise the boiling point of the solvent. Adding salt to boil water when cooking ...
... solute to a liquid solvent will usually raise the boiling point of the solvent. Adding salt to boil water when cooking ...
sch3u unit 1 test: matter
... c. The same elements are present in all fluorescent lights. d. Air is present in all fluorescent lights. 4. Choose the pair of elements that will form a compound with the most ionic character a. Li & O b. C & O c. F & F d. Cl & Br 5. In the alkali metals group, as the atomic number increases a. the ...
... c. The same elements are present in all fluorescent lights. d. Air is present in all fluorescent lights. 4. Choose the pair of elements that will form a compound with the most ionic character a. Li & O b. C & O c. F & F d. Cl & Br 5. In the alkali metals group, as the atomic number increases a. the ...
AP Chemistry - School Webmasters
... 36. The molecular formula of morphine, a pain-killing narcotic, is C17H19NO3. a. What is the molar mass? b. What fraction of atoms in morphine is accounted for by carbon? c. Which element contributes least to the molar mass? 37. Complete the list of ionic compounds ( name or formula) a. Cupric Hydro ...
... 36. The molecular formula of morphine, a pain-killing narcotic, is C17H19NO3. a. What is the molar mass? b. What fraction of atoms in morphine is accounted for by carbon? c. Which element contributes least to the molar mass? 37. Complete the list of ionic compounds ( name or formula) a. Cupric Hydro ...
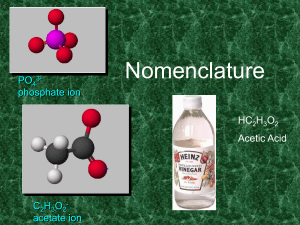
Nomenclature
... Names of Variable Ions These elements REQUIRE Roman Numerals because they can have more than one possible charge: anything except Group 1A, 2A, Ag, Zn, Cd, and Al (You should already know the charges on these!) ...
... Names of Variable Ions These elements REQUIRE Roman Numerals because they can have more than one possible charge: anything except Group 1A, 2A, Ag, Zn, Cd, and Al (You should already know the charges on these!) ...
Summary notes - Kelso High School
... Acids and pH The word ACID means sour. We often say that a sour liquid tastes acid. Examples of sour liquids that you will find in the home are vinegar, lemon juice, grapefruit juice and sour milk. You can find out if a substance is an acid or and alkali by dissolving it in water and adding an indi ...
... Acids and pH The word ACID means sour. We often say that a sour liquid tastes acid. Examples of sour liquids that you will find in the home are vinegar, lemon juice, grapefruit juice and sour milk. You can find out if a substance is an acid or and alkali by dissolving it in water and adding an indi ...
Chemical Equation Reactions
... Active metals replace less active metals from their compounds in aqueous solution. Magnesium turnings are added to a solution of iron(III) chloride. Active metals replace hydrogen in water. Sodium is added to water. Active metals replace hydrogen in acids. Lithium is added to hydrochloric acid ...
... Active metals replace less active metals from their compounds in aqueous solution. Magnesium turnings are added to a solution of iron(III) chloride. Active metals replace hydrogen in water. Sodium is added to water. Active metals replace hydrogen in acids. Lithium is added to hydrochloric acid ...
CHEMISTRY
... elements to combine and form compounds depends on the number and arrangement of electrons in their outermost energy level • Atoms are most stable when their outer most energy level is full ...
... elements to combine and form compounds depends on the number and arrangement of electrons in their outermost energy level • Atoms are most stable when their outer most energy level is full ...
Formulae/ Equations homework - St Peter the Apostle High School
... copper carbonate powder is heated in a test tube. (e) In the intestines the starch in our food is broken down to form glucose and water. (f) Hydrogen iodide is an unstable liquid that spontaneously decomposes to form water and oxygen gas. ...
... copper carbonate powder is heated in a test tube. (e) In the intestines the starch in our food is broken down to form glucose and water. (f) Hydrogen iodide is an unstable liquid that spontaneously decomposes to form water and oxygen gas. ...
Exam Review
... d) propane + oxygen carbon dioxide + water (C) e) lead(II) hydroxide lead(II) oxide + water (D) f) ammonia + sulphuric acid ammonium sulphate (S) g) potassium phosphate + magnesium chloride magnesium phosphate + potassium chloride (DD) 6. For each of the following, use an activity series to ...
... d) propane + oxygen carbon dioxide + water (C) e) lead(II) hydroxide lead(II) oxide + water (D) f) ammonia + sulphuric acid ammonium sulphate (S) g) potassium phosphate + magnesium chloride magnesium phosphate + potassium chloride (DD) 6. For each of the following, use an activity series to ...
Are You suprised ?
... c. P2F4 _______________________________________ d. SeO3 _______________________________________ 4. Draw the following molecular structures for the covalent compounds. 5. a. SeBr2 ...
... c. P2F4 _______________________________________ d. SeO3 _______________________________________ 4. Draw the following molecular structures for the covalent compounds. 5. a. SeBr2 ...
Ch. 9
... – For metals that can have more than one charge, the name of the metal is succeeded by the valency in capital Roman numerals in () parentheses OR by using the suffix –ous for the lowest valency & -ic for the highest valency ...
... – For metals that can have more than one charge, the name of the metal is succeeded by the valency in capital Roman numerals in () parentheses OR by using the suffix –ous for the lowest valency & -ic for the highest valency ...
Chap. 4 - Chemical Reactions
... Active metals replace less active metals from their compounds in aqueous solution. Magnesium turnings are added to a solution of iron(III) chloride. Active metals replace hydrogen in water. Sodium is added to water. Active metals replace hydrogen in acids. Lithium is added to hydrochloric acid ...
... Active metals replace less active metals from their compounds in aqueous solution. Magnesium turnings are added to a solution of iron(III) chloride. Active metals replace hydrogen in water. Sodium is added to water. Active metals replace hydrogen in acids. Lithium is added to hydrochloric acid ...
chapter 4 review: types of chemical reactions and
... 8. 2 H2O + 4 MnO4- + 3 ClO2- 4 MnO2 + 3 ClO4- + 4OHWhich species acts as an oxidizing agent in the reaction represented above? (a) H2O (b) ClO4(c) ClO2(d) MnO2 (e) MnO49. The volume of distilled water that should be added to 10.0 mL of 6.00 M HCl (aq) in order to prepare a 0.500 M HCl (aq)solution ...
... 8. 2 H2O + 4 MnO4- + 3 ClO2- 4 MnO2 + 3 ClO4- + 4OHWhich species acts as an oxidizing agent in the reaction represented above? (a) H2O (b) ClO4(c) ClO2(d) MnO2 (e) MnO49. The volume of distilled water that should be added to 10.0 mL of 6.00 M HCl (aq) in order to prepare a 0.500 M HCl (aq)solution ...
1A - The changing atom History of the atom • The model of the atom
... These are chemical opposites to acids which include: metal oxides, hydroxides, ammonia and amines. They neutralise acids Bases can be defined as proton acceptors Alkalis These compounds dissolved in water gives a solution with a pH of greater than 7 Some common alkalis: sodium hydroxide, pot ...
... These are chemical opposites to acids which include: metal oxides, hydroxides, ammonia and amines. They neutralise acids Bases can be defined as proton acceptors Alkalis These compounds dissolved in water gives a solution with a pH of greater than 7 Some common alkalis: sodium hydroxide, pot ...
Instructions for AP/IB 2 Chem Summer Assignment Note
... There are 6 strong acids (these dissociate completely in aqueous solution; ex: HCl exists as H+ and Cl- ions, not as molecules) Memorize the six strong acids: HCl, HBr, HI, HNO3, H2SO4, HClO4 ...
... There are 6 strong acids (these dissociate completely in aqueous solution; ex: HCl exists as H+ and Cl- ions, not as molecules) Memorize the six strong acids: HCl, HBr, HI, HNO3, H2SO4, HClO4 ...
Name__________________________ Period_______ Word
... example of a reaction condition is the heat symbol (∆ ), which indicates that the reactants were heated. An equation can also indicate the physical state of the reactants and products. The physical states of matter symbols are listed below. ...
... example of a reaction condition is the heat symbol (∆ ), which indicates that the reactants were heated. An equation can also indicate the physical state of the reactants and products. The physical states of matter symbols are listed below. ...
Gas-forming Reactions
... the peroxide ion (O22–) in which its oxidation state is - 1, 4. Hydrogen almost always has an oxidation state of +1. Exceptions include metal hydrides (such as NaH) in which its oxidation state is -1. 5. Fluorine (as an ion) always has an oxidation state of – 1. No exceptions. 6. The other halogens ...
... the peroxide ion (O22–) in which its oxidation state is - 1, 4. Hydrogen almost always has an oxidation state of +1. Exceptions include metal hydrides (such as NaH) in which its oxidation state is -1. 5. Fluorine (as an ion) always has an oxidation state of – 1. No exceptions. 6. The other halogens ...
SAMPLE PAPER Class - XII SUBJECT
... Q.11. Calculate the freezing point of one molar aq. solution(density 1.04gcm-3)of KCl. [Kf for water =1.86Kkgmol-1,At.Mass K=39,Cl=35.5] Q.12. A element crystallizes in a simple cubic structure. Calculate the unit cell edge length when its density 8 gm/cm3 if 200 gm of this element contains 24 x 102 ...
... Q.11. Calculate the freezing point of one molar aq. solution(density 1.04gcm-3)of KCl. [Kf for water =1.86Kkgmol-1,At.Mass K=39,Cl=35.5] Q.12. A element crystallizes in a simple cubic structure. Calculate the unit cell edge length when its density 8 gm/cm3 if 200 gm of this element contains 24 x 102 ...
Naming Binary Inorganic Compounds
... Iron (III) chloride Naming of Compounds that contain Polyatomic Ions Polyatomic ions are ___________ molecules. The atoms within a polyatomic ion are usually very tightly bound together, so the ion retains its identity within ionic compounds and over the course of many chemical reactions. o o o o ...
... Iron (III) chloride Naming of Compounds that contain Polyatomic Ions Polyatomic ions are ___________ molecules. The atoms within a polyatomic ion are usually very tightly bound together, so the ion retains its identity within ionic compounds and over the course of many chemical reactions. o o o o ...
Stoichiometry - Cloudfront.net
... only carbon, hydrogen, and oxygen, determine the empirical formula of the unknown compound. 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 mg H2O on combustion. The compound contains 4.680% N by mass. Is the white soli ...
... only carbon, hydrogen, and oxygen, determine the empirical formula of the unknown compound. 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 mg H2O on combustion. The compound contains 4.680% N by mass. Is the white soli ...
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.The corresponding electrically neutral compound •HO is the hydroxyl radical. The corresponding covalently-bound group -OH of atoms is the hydroxyl group.Hydroxide ion and hydroxyl group are nucleophiles and can act as a catalyst in organic chemistry.Many inorganic substances which bear the word ""hydroxide"" in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxyl groups.