Dr. Spencer`s PPT
... – formation of an insoluble solid – formation of either a soluble weak electrolyte or soluble nonelectrolyte – formation of a gas which escapes from solution Chapt. 4.5 Chem 106, Prof. J.T. Spencer ...
... – formation of an insoluble solid – formation of either a soluble weak electrolyte or soluble nonelectrolyte – formation of a gas which escapes from solution Chapt. 4.5 Chem 106, Prof. J.T. Spencer ...
Chemistry notes Important terms *Mass of element in a sample
... *Mass of element in a sample Periods- horizontal rows on a periodic table Groups- vertical rows on a periodic table Ionic compound – electron transfer from a metal to a non-metal Covalent compound- electron sharing between two non-metals Hydrates- have a specific number of water molecules associated ...
... *Mass of element in a sample Periods- horizontal rows on a periodic table Groups- vertical rows on a periodic table Ionic compound – electron transfer from a metal to a non-metal Covalent compound- electron sharing between two non-metals Hydrates- have a specific number of water molecules associated ...
aq - Byron High School
... substances (see rules) (aq) – soluble substances (see rules) Aqueous Reactions ...
... substances (see rules) (aq) – soluble substances (see rules) Aqueous Reactions ...
Chapter 4
... Table 4.1 lists examples of strong electrolytes, weak electrolytes, and nonelectrolytes. Ionic compounds, such as sodium chloride, potassium iodide (KI), and calcium nitrate [Ca(NO3)2], are strong electrolytes. It is interesting to note that human body fluids contain many strong and weak electrolyte ...
... Table 4.1 lists examples of strong electrolytes, weak electrolytes, and nonelectrolytes. Ionic compounds, such as sodium chloride, potassium iodide (KI), and calcium nitrate [Ca(NO3)2], are strong electrolytes. It is interesting to note that human body fluids contain many strong and weak electrolyte ...
Chemical Reactions
... If you follow these alterations, it will be easy to identify this as a redox reaction and visualize the electron transfer. The simplest way to follow the alteration is to assign oxidation states to the elements that undergo the change. There are two rules of importance associated with single displac ...
... If you follow these alterations, it will be easy to identify this as a redox reaction and visualize the electron transfer. The simplest way to follow the alteration is to assign oxidation states to the elements that undergo the change. There are two rules of importance associated with single displac ...
Unit 8: Reactions - Mark Rosengarten
... oxygen! Or if the salt on your plate decomposed suddenly into sodium (explosive metal) and chlorine (poisonous, corrosive gas)! Compounds exist because it requires less energy to exist in compound form. This is why the diatomic molecules exist>hydrogen has less energy as H2 than as just H>so wheneve ...
... oxygen! Or if the salt on your plate decomposed suddenly into sodium (explosive metal) and chlorine (poisonous, corrosive gas)! Compounds exist because it requires less energy to exist in compound form. This is why the diatomic molecules exist>hydrogen has less energy as H2 than as just H>so wheneve ...
File - Junior College Chemistry tuition
... An organic liquid Q with molecular formula C5H10O2, shows a broad absorption at 3100–3500cm–1 in the infra–red spectrum. When Q reacts with acidified sodium dichromate(VI) solution under mild conditions, a liquid can be distilled from the reaction mixture. This liquid gives a brick–red precipitate o ...
... An organic liquid Q with molecular formula C5H10O2, shows a broad absorption at 3100–3500cm–1 in the infra–red spectrum. When Q reacts with acidified sodium dichromate(VI) solution under mild conditions, a liquid can be distilled from the reaction mixture. This liquid gives a brick–red precipitate o ...
Transition Metals
... Zn can only form a +2 ion. In this ion the Zn2+ has a complete d orbital and so does not meet the criteria of having a incomplete d orbital in one of its compounds. Sc only forms a +3 ion with the electronic structure The Sc3+ ion had an empty d orbital and so also does not meet the criteria ...
... Zn can only form a +2 ion. In this ion the Zn2+ has a complete d orbital and so does not meet the criteria of having a incomplete d orbital in one of its compounds. Sc only forms a +3 ion with the electronic structure The Sc3+ ion had an empty d orbital and so also does not meet the criteria ...
Coordination and Chemistry of Stable Cu (II) Complexes in the Gas
... Ag(II),26 Au(II),27 Mn(II),28 Pb(II),28 Cr(II),28 and Ho(III)29 in association with a very wide range of ligands. In several instances, for example, [Mg·(thf)4]2+ and [Ag·(pyridine)4]2+,22,26 it has been observed that ions identified as the most stable metal-ligand configurations in the gas phase ar ...
... Ag(II),26 Au(II),27 Mn(II),28 Pb(II),28 Cr(II),28 and Ho(III)29 in association with a very wide range of ligands. In several instances, for example, [Mg·(thf)4]2+ and [Ag·(pyridine)4]2+,22,26 it has been observed that ions identified as the most stable metal-ligand configurations in the gas phase ar ...
Review: The preparation of Hydantoins
... more advantageous to convert methyleneamino-acetonitrile in aminoacetonitrile hydrosulfate to implement this idea with potassium cyanate, which is added to the complete saturation of the sulfuric acid 0.5 mol of sodium carbonate, to saponify the acetonitrile-urea to fuse and arrive at hydantoin. In ...
... more advantageous to convert methyleneamino-acetonitrile in aminoacetonitrile hydrosulfate to implement this idea with potassium cyanate, which is added to the complete saturation of the sulfuric acid 0.5 mol of sodium carbonate, to saponify the acetonitrile-urea to fuse and arrive at hydantoin. In ...
SOLUBILITY RULES FOR IONIC COMPOUNDS IN WATER
... (b) Calculate Kc for this reaction at 503 K, with proper units. (c) Calculate Kp for this reaction at 503 K, with proper units. 2. Ammonia decomposes according to the reaction: 2NH3 (g) ⇆ N2 (g) + 3H2 (g) A 2.00 liter tank is originally charged with 0.500 moles of ammonia, and at equilibrium it is f ...
... (b) Calculate Kc for this reaction at 503 K, with proper units. (c) Calculate Kp for this reaction at 503 K, with proper units. 2. Ammonia decomposes according to the reaction: 2NH3 (g) ⇆ N2 (g) + 3H2 (g) A 2.00 liter tank is originally charged with 0.500 moles of ammonia, and at equilibrium it is f ...
Pirogov National Medical Univercity of Vinnitsa
... 4. Combustible, flammable and volatile substances can not be stored close to the flame or very hot electric devices (thermostats, electronic, etc.) . 5. Alkali metals should always be kept in a layer of inert against water and moisture kerosene. Alkali metals and crystalline alkali should be taken o ...
... 4. Combustible, flammable and volatile substances can not be stored close to the flame or very hot electric devices (thermostats, electronic, etc.) . 5. Alkali metals should always be kept in a layer of inert against water and moisture kerosene. Alkali metals and crystalline alkali should be taken o ...
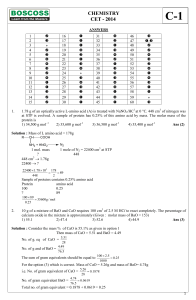
CHEMISTRY CET
... The statement that is NOT correct is 1) Van der Waals constant 'a' measures extent of intermolecular attractive forces for real gases. (2) Boyle point depends on the nature of real gas. (3) Compressibility factor measures the deviation of real gas from ideal behaviour. (4) Critical temperature is th ...
... The statement that is NOT correct is 1) Van der Waals constant 'a' measures extent of intermolecular attractive forces for real gases. (2) Boyle point depends on the nature of real gas. (3) Compressibility factor measures the deviation of real gas from ideal behaviour. (4) Critical temperature is th ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... Hydrated sodium and chloride ions Notice that in Interactive Figure 4.2.1 the water molecules orient themselves so that the oxygen atoms are near the Na+ cations and the hydrogen atoms are near the Cl anions. This is due to the polar nature of water, a result of uneven electron distribution in wat ...
... Hydrated sodium and chloride ions Notice that in Interactive Figure 4.2.1 the water molecules orient themselves so that the oxygen atoms are near the Na+ cations and the hydrogen atoms are near the Cl anions. This is due to the polar nature of water, a result of uneven electron distribution in wat ...
Chemical Reactions and Solution Stoichiometry
... cations and anions held together by ionic forces in a three-dimensional arrangement. When a soluble ionic compound dissolves in water, strong hydration forces between ions and water molecules replace these ionic forces. If the ionic forces are very strong, however, the compound does not dissociate i ...
... cations and anions held together by ionic forces in a three-dimensional arrangement. When a soluble ionic compound dissolves in water, strong hydration forces between ions and water molecules replace these ionic forces. If the ionic forces are very strong, however, the compound does not dissociate i ...
Liquid chromatography: a tool for the analysis of metal species
... acids (from mono-, di-, tricarboxylic acids to chelating agents such as a-hydroxyisobutyric acid, tartaric, citric, oxalic, pyridine-2,6-dicarboxylic acid, 1,2diaminocyclohexanetetraacetic acid, diethylenetriaminopentaacetic acid) have been evaluated for the simultaneous ion-chromatography of anions ...
... acids (from mono-, di-, tricarboxylic acids to chelating agents such as a-hydroxyisobutyric acid, tartaric, citric, oxalic, pyridine-2,6-dicarboxylic acid, 1,2diaminocyclohexanetetraacetic acid, diethylenetriaminopentaacetic acid) have been evaluated for the simultaneous ion-chromatography of anions ...
Chem 1B Fa2015 FinalExam Review
... The decomposition of N2O5 according the equation: 2 N2O5(g) 4 NO2(g) + O2(g), follows a first order rate law, such that: Rate = k[N2O5]. When the reaction was carried out at a certain temperature using an initial concentration [N2O5]0 = 0.100 M, the concentration of N2O5 after 5.00 minutes (300 se ...
... The decomposition of N2O5 according the equation: 2 N2O5(g) 4 NO2(g) + O2(g), follows a first order rate law, such that: Rate = k[N2O5]. When the reaction was carried out at a certain temperature using an initial concentration [N2O5]0 = 0.100 M, the concentration of N2O5 after 5.00 minutes (300 se ...
Exam 1
... • make sure chemical equations are balanced and that the formulas for individual substances include an indication of state; for example, H2 (g); NaCl (s). ...
... • make sure chemical equations are balanced and that the formulas for individual substances include an indication of state; for example, H2 (g); NaCl (s). ...
IONIC EQULIBRIUM
... Electrolyte. A compound which produces positive and negative ions in solution. Strong electrolytes are completely dissociated, whereas weak electrolytes are only partially dissociated. Hydrolysis. An acid-base reaction of a cation or anion with water. Isoelectric point. The pH at which there is an e ...
... Electrolyte. A compound which produces positive and negative ions in solution. Strong electrolytes are completely dissociated, whereas weak electrolytes are only partially dissociated. Hydrolysis. An acid-base reaction of a cation or anion with water. Isoelectric point. The pH at which there is an e ...
Complete Set
... increasing levels of CO2 will lead to increased dissolution of CaCO3 and critically affect the survival of life forms that rely on a carbonaceous skeleton. Calculate the concentrations of Ca2+ and CO32– in a saturated solution of CaCO3. (The Ksp of CaCO3 is 3.3 × 10–9.) The dissolution of CaCO3 foll ...
... increasing levels of CO2 will lead to increased dissolution of CaCO3 and critically affect the survival of life forms that rely on a carbonaceous skeleton. Calculate the concentrations of Ca2+ and CO32– in a saturated solution of CaCO3. (The Ksp of CaCO3 is 3.3 × 10–9.) The dissolution of CaCO3 foll ...
M for Moles - Shop
... To date, there are around 120 known types of atoms or elements. Of these, about 90 elements can be found in nature. All matters are made of these elements. The rest, usually those of heavier ones (from uranium with atomic number 92 onwards) no longer exist or are found only in traces. However, these ...
... To date, there are around 120 known types of atoms or elements. Of these, about 90 elements can be found in nature. All matters are made of these elements. The rest, usually those of heavier ones (from uranium with atomic number 92 onwards) no longer exist or are found only in traces. However, these ...
____ 1. The energy required to convert a ground
... 38. Gases W and X react in a closed, rigid vessel to form gases Y and Z according to the equation above. The intial pressure of W(g) is 1.20 atm and that of X(g) is 1.60 atm. No Y(g) or Z(g) is initially present. The experiment is carried out at constant temperature. What is the partial pressure of ...
... 38. Gases W and X react in a closed, rigid vessel to form gases Y and Z according to the equation above. The intial pressure of W(g) is 1.20 atm and that of X(g) is 1.60 atm. No Y(g) or Z(g) is initially present. The experiment is carried out at constant temperature. What is the partial pressure of ...
Fundamentals Diagnostic Quiz
... 44. How many moles of sodium ions are there in 2.5 moles of sodium chloride, NaCl? a) 6.0221 x 1023 moles b) 1.5055 x 102 moles c) 1.0 mole d) 2.5 moles e) 5.0 moles ...
... 44. How many moles of sodium ions are there in 2.5 moles of sodium chloride, NaCl? a) 6.0221 x 1023 moles b) 1.5055 x 102 moles c) 1.0 mole d) 2.5 moles e) 5.0 moles ...
AQA GCSE Chemistry My Revision Notes
... (c) Suggest how you would remove excess copper oxide when the reaction is complete. (1 mark) (d) What process would you use to produce solid copper sulfate crystals from the solution? (1 mark) (e) What is the name given to a reaction in which an acid reacts with a base to make a salt? (1 mark) (f) H ...
... (c) Suggest how you would remove excess copper oxide when the reaction is complete. (1 mark) (d) What process would you use to produce solid copper sulfate crystals from the solution? (1 mark) (e) What is the name given to a reaction in which an acid reacts with a base to make a salt? (1 mark) (f) H ...
W1 WORKSHOP ON STOICHIOMETRY
... When an ionic solid dissolves in water to form a solution, the charges on the ions are indicated. NaCl(s) → Na+(aq) + Cl–(aq) Again, the atoms must balance. Notice also that the electrical charges present on both sides of the equation must balance as well. In another example: BaCl2(s) → Ba2+(aq) + 2 ...
... When an ionic solid dissolves in water to form a solution, the charges on the ions are indicated. NaCl(s) → Na+(aq) + Cl–(aq) Again, the atoms must balance. Notice also that the electrical charges present on both sides of the equation must balance as well. In another example: BaCl2(s) → Ba2+(aq) + 2 ...
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.The corresponding electrically neutral compound •HO is the hydroxyl radical. The corresponding covalently-bound group -OH of atoms is the hydroxyl group.Hydroxide ion and hydroxyl group are nucleophiles and can act as a catalyst in organic chemistry.Many inorganic substances which bear the word ""hydroxide"" in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxyl groups.