The p-Block Elements The p-Block Elements
... boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. The ammonia molecule is trigonal pyramidal ...
... boiling points are 198.4 and 239.7 K respectively. In the solid and liquid states, it is associated through hydrogen bonds as in the case of water and that accounts for its higher melting and boiling points than expected on the basis of its molecular mass. The ammonia molecule is trigonal pyramidal ...
AP Chem Summer Assignment KEY
... need your own paper for parts of it. – show your work and clearly mark your final answers!! Use correct significant figures for math answers and units where needed! Arrive at Stadium with all of these items completed and you will be well on your way to a terrific year in AP Chemistry! ...
... need your own paper for parts of it. – show your work and clearly mark your final answers!! Use correct significant figures for math answers and units where needed! Arrive at Stadium with all of these items completed and you will be well on your way to a terrific year in AP Chemistry! ...
Journal of Molecular Catalysis A, 302
... Table 1 summarizes the results of scoping experiments in which some components of the reaction mixture were omitted in order to determine their importance. Entries 1 and 2 show standard reaction conditions for Rh- and Pd-catalyzed reactions, respectively. The observed products in all cases were 4-tr ...
... Table 1 summarizes the results of scoping experiments in which some components of the reaction mixture were omitted in order to determine their importance. Entries 1 and 2 show standard reaction conditions for Rh- and Pd-catalyzed reactions, respectively. The observed products in all cases were 4-tr ...
welcome to ap chemistry - Garnet Valley School District
... cover the basics of chemistry, which are covered in chapters 1, 2, 3 and 4 of the textbook: Chemistry: A Molecular Approach by Nivaldo J. Tro. The topics covered are chemical formulas, equation writing and balancing, formula and reaction stoichiometry, gas laws and solutions. This will be review for ...
... cover the basics of chemistry, which are covered in chapters 1, 2, 3 and 4 of the textbook: Chemistry: A Molecular Approach by Nivaldo J. Tro. The topics covered are chemical formulas, equation writing and balancing, formula and reaction stoichiometry, gas laws and solutions. This will be review for ...
Otto F. Meyerhof - Nobel Lecture
... the heat occurs in the recovery phase. For if out of six molecules, for instance, only one is burnt then there must arose from the conversion of 1 g of sugar 3772/6 = 630 cal, of which 385 are in the fatigue phase, so that 245 must be in the recovery phase. In this case 60% must already be released ...
... the heat occurs in the recovery phase. For if out of six molecules, for instance, only one is burnt then there must arose from the conversion of 1 g of sugar 3772/6 = 630 cal, of which 385 are in the fatigue phase, so that 245 must be in the recovery phase. In this case 60% must already be released ...
LECTURE_Solutions2013(1)
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
... • C12H22O11 (s) C12H22O11 (aq) • NO dissociation because NO ions • Sucrose dissolves in water because sugar is polar (-OH group), but dissociation does not occur. Sucrose molecules are simply separated from each other. No ions are formed ...
environmental science & services - level 1
... the atmosphere is not directly influenced by human activity. However, its concentration is influenced indirectly as any increase in global temperatures will increase concentrations, as warmer air can hold more water. ...
... the atmosphere is not directly influenced by human activity. However, its concentration is influenced indirectly as any increase in global temperatures will increase concentrations, as warmer air can hold more water. ...
Equation Writing Information
... On the AP examination you will encounter a question in which you will be required to write net ionic equations for various reactions. In past years, students have been required to choose 5 of 8 reactions. Some of the reactions you will undoubtedly recognize; others you will not! Hopefully, at least ...
... On the AP examination you will encounter a question in which you will be required to write net ionic equations for various reactions. In past years, students have been required to choose 5 of 8 reactions. Some of the reactions you will undoubtedly recognize; others you will not! Hopefully, at least ...
Chapter 4 Notes: Types of Reactions & Solution
... •For instance, hydrogen chloride molecules, which are polar, give up their hydrogens to water, •forming chloride ions (Cl-) •and hydronium ions (H3O+). ...
... •For instance, hydrogen chloride molecules, which are polar, give up their hydrogens to water, •forming chloride ions (Cl-) •and hydronium ions (H3O+). ...
Advanced Placement Chemistry
... (A) 0.20 m C6H12O6, glucose (B) 0.20 m NH4Br (C) 0.20 m ZnSO4 (D) 0.20 m KMnO4 (E) 0.20 m MgCl2 45. A sample of an ideal gas is cooled from 50.0 °C to 25.0 °C in a sealed container of constant volume. Which of the following values for the gas will decrease? I. The average molecular mass of the gas ...
... (A) 0.20 m C6H12O6, glucose (B) 0.20 m NH4Br (C) 0.20 m ZnSO4 (D) 0.20 m KMnO4 (E) 0.20 m MgCl2 45. A sample of an ideal gas is cooled from 50.0 °C to 25.0 °C in a sealed container of constant volume. Which of the following values for the gas will decrease? I. The average molecular mass of the gas ...
MC94 - Southchemistry.com
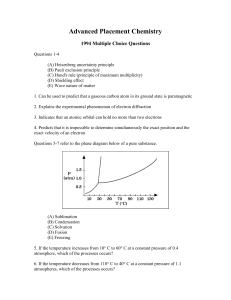
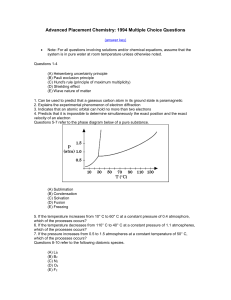
... Advanced Placement Chemistry: 1994 Multiple Choice Questions (answer key) ...
... Advanced Placement Chemistry: 1994 Multiple Choice Questions (answer key) ...
A2 2, Analytical, Transition Metals, Electrochemistry and
... breathing carbon monoxide can result in death. ...
... breathing carbon monoxide can result in death. ...
oxidation and reduction
... a) Oxidation and reduction are brought about by substances referred to as oxidising agents and reducing agents. Define each of these substances in terms of electrons. Oxidising agent ..................................................................................................................... ...
... a) Oxidation and reduction are brought about by substances referred to as oxidising agents and reducing agents. Define each of these substances in terms of electrons. Oxidising agent ..................................................................................................................... ...
2010 - SAASTA
... atom surrounded by 4 oxygen atoms. These silicon-oxygen tetrahedrons are joined together by a network of bonds that makes the mineral extremely strong. 26. Which one of the following is not a fossil fuel? A. Coal B. Crude oil C. Natural gas D. None of the above Answer: D A fossil fuel is any of a cl ...
... atom surrounded by 4 oxygen atoms. These silicon-oxygen tetrahedrons are joined together by a network of bonds that makes the mineral extremely strong. 26. Which one of the following is not a fossil fuel? A. Coal B. Crude oil C. Natural gas D. None of the above Answer: D A fossil fuel is any of a cl ...
Exam 1
... D. less than 2 mol Question 20 In a second exercise, the same initial amounts of reactants are mixed at 600 K in a well-insulated container of the same size. In this case, the amount of sulfur trioxide present when the mixture reaches equilibrium would be A. equal to the amount produced in the previ ...
... D. less than 2 mol Question 20 In a second exercise, the same initial amounts of reactants are mixed at 600 K in a well-insulated container of the same size. In this case, the amount of sulfur trioxide present when the mixture reaches equilibrium would be A. equal to the amount produced in the previ ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.