2014_S4_CHM_NORMAL (ALL)
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
... 53. Element X (atomic number 11) reacts with element Y (atomic number 16) to form an ionic compound. Each atom of X loses one electron and each atom of Y accepts two electrons to form a compound with formula X2Y. 54. Consider the following information: ...
BC Science 10 Workbook Answers
... bacteria, using a series of chemical reactions, convert nitrate back into nitrogen gas. 8. Eutrophication is the process by which excess nutrients result in increased plant production and decay in aquatic ecosystems. Interpreting Illustrations ...
... bacteria, using a series of chemical reactions, convert nitrate back into nitrogen gas. 8. Eutrophication is the process by which excess nutrients result in increased plant production and decay in aquatic ecosystems. Interpreting Illustrations ...
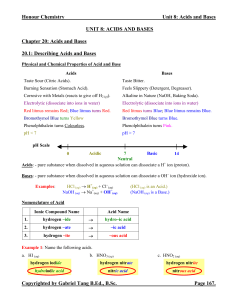
8.4 Weak Acids and Bases, Continued
... The Autoionization of Water, Kw • Water can act as either a weak acid or a base depending on whether a base or acid is present in solution. • In pure water, the water molecules spontaneously react with each other as shown. ...
... The Autoionization of Water, Kw • Water can act as either a weak acid or a base depending on whether a base or acid is present in solution. • In pure water, the water molecules spontaneously react with each other as shown. ...
Dr David`s Chemistry Test Answers
... 2. Many familiar inorganic reactions like the reaction of hydrochloric acid with sodium hydroxide are not reversible. Similarly, the reaction of silver nitrate solution with sodium chloride solution is a reaction which only goes one way (ie, left to right). This produces a white precipitate of silve ...
... 2. Many familiar inorganic reactions like the reaction of hydrochloric acid with sodium hydroxide are not reversible. Similarly, the reaction of silver nitrate solution with sodium chloride solution is a reaction which only goes one way (ie, left to right). This produces a white precipitate of silve ...
Acid-Base Reactions Worksheet #2 - Mro
... 1. Cheating on a test will result in a zero for a major exam. Plagiarizing a lab report will result in a zero for that lab report. 2. Cutting class will result in a zero for any test or lab report expected for that class day. In addition, I will require a note from your parents informing me that the ...
... 1. Cheating on a test will result in a zero for a major exam. Plagiarizing a lab report will result in a zero for that lab report. 2. Cutting class will result in a zero for any test or lab report expected for that class day. In addition, I will require a note from your parents informing me that the ...
Basic Concepts - Department of Chemistry
... 2. If reactant side has more moles of gas a. Increase in denominator is greater than increase in numerator and Qc < Kc b. To return to equilibrium, Qc must increase; the numerator of the Qc expression must increase and denominator must decrease—it shifts toward fewer moles of gas (reactants to produ ...
... 2. If reactant side has more moles of gas a. Increase in denominator is greater than increase in numerator and Qc < Kc b. To return to equilibrium, Qc must increase; the numerator of the Qc expression must increase and denominator must decrease—it shifts toward fewer moles of gas (reactants to produ ...
chemical change
... The evidence for a chemical reaction occurring, is the formation of a substance which is different from the original reactant or reactant, this is often accompanied by changes in energy, which are measured as temperature changes. Thus for the reaction of the silver metal sodium with the green/yellow ...
... The evidence for a chemical reaction occurring, is the formation of a substance which is different from the original reactant or reactant, this is often accompanied by changes in energy, which are measured as temperature changes. Thus for the reaction of the silver metal sodium with the green/yellow ...
Acid rain
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it possesses elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Governments have made efforts since the 1970s to reduce the release of sulfur dioxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes and sulfur dioxide is produced by volcanic eruptions. The chemicals in acid rain can cause paint to peel, corrosion of steel structures such as bridges, and erosion of stone statues.