
3.1.1.4 Proteins
... muscle proteins that work together to cause a muscle to contract. There are proteins in cell membranes that help identify a cell or serve as a receptor. Adrenalin and insulin are two examples of hormones that are made of protein. All proteins have a special shape that is the result of the interactio ...
... muscle proteins that work together to cause a muscle to contract. There are proteins in cell membranes that help identify a cell or serve as a receptor. Adrenalin and insulin are two examples of hormones that are made of protein. All proteins have a special shape that is the result of the interactio ...
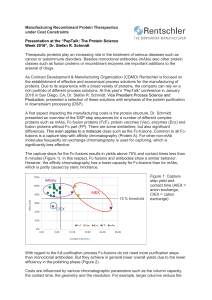
Manufacturing Recombinant Protein Therapeutics under Cost
... in downstream processing (DSP). A first aspect impacting the manufacturing costs is the protein structure. Dr. Schmidt presented an overview of the DSP step sequences for a number of different complex proteins such as mAbs, Fc-fusion proteins (FcF), protein vaccines (Vac), enzymes (Enz) and fusion p ...
... in downstream processing (DSP). A first aspect impacting the manufacturing costs is the protein structure. Dr. Schmidt presented an overview of the DSP step sequences for a number of different complex proteins such as mAbs, Fc-fusion proteins (FcF), protein vaccines (Vac), enzymes (Enz) and fusion p ...
Proteins - Science Learning Hub
... Context > Food Function and Structure > Teaching and Learning Approaches > Proteins ...
... Context > Food Function and Structure > Teaching and Learning Approaches > Proteins ...
In general, animal proteins are considered complete proteins. A complete... essential amino acids. Vegetable (plant-based) proteins are considered incomplete proteins...
... what protein sources you eat. A vegetarian can acquire the recommended amount of protein with a method known as complimentary protein, where you combine certain foods that will create a complete protein. For more information email: nutritionist@wcupa.edu ...
... what protein sources you eat. A vegetarian can acquire the recommended amount of protein with a method known as complimentary protein, where you combine certain foods that will create a complete protein. For more information email: nutritionist@wcupa.edu ...
Protein folding
... interactions responsible for the higher order structures. Destruction of the higher order structure is leads to the loss of activity of a protein. This process is referred as denaturation or unfolding of protein. ...
... interactions responsible for the higher order structures. Destruction of the higher order structure is leads to the loss of activity of a protein. This process is referred as denaturation or unfolding of protein. ...
Protein structure
... tertiary or quaternary structures are usually referred to as chemical conformation, and transitions between them are called conformational changes. The primary structure is held together by covalent or peptide bonds, which are made during the process of protein biosynthesis or translation. These pep ...
... tertiary or quaternary structures are usually referred to as chemical conformation, and transitions between them are called conformational changes. The primary structure is held together by covalent or peptide bonds, which are made during the process of protein biosynthesis or translation. These pep ...
In order to carry out their functions, proteins need to move. Scientists
... developing drugs that can efficiently interact with it. But because of its complexity, protein motion has been notoriously difficult to study. Scientists at IBS‐Grenoble, EPFL and ENS‐Lyon, have developed a new method for studying protein motion by first freezing proteins and then slowly “waking ...
... developing drugs that can efficiently interact with it. But because of its complexity, protein motion has been notoriously difficult to study. Scientists at IBS‐Grenoble, EPFL and ENS‐Lyon, have developed a new method for studying protein motion by first freezing proteins and then slowly “waking ...
Abstracts
... pathogens. Not only exogenous molecules, but also components of plasma membranes are internalized by endocytosis to achieve cellular functions. For example, cells internalize receptors at the cell surface by endocytosis to “down regulate” sensitivities to ligand molecules to the receptors. Another e ...
... pathogens. Not only exogenous molecules, but also components of plasma membranes are internalized by endocytosis to achieve cellular functions. For example, cells internalize receptors at the cell surface by endocytosis to “down regulate” sensitivities to ligand molecules to the receptors. Another e ...
Biochemical studies on animal models of ceroid
... This thesis presents further characterisation and identification of the stored ovine lipopigment proteins. Separation of the lipopigment proteins by LDS-PAGE showed the presence of the 3.5 kDa and 14.8 kDa proteins noted in earlier studies, and an additional band at 24 kDa. The 14.8 and 24 kDa bands ...
... This thesis presents further characterisation and identification of the stored ovine lipopigment proteins. Separation of the lipopigment proteins by LDS-PAGE showed the presence of the 3.5 kDa and 14.8 kDa proteins noted in earlier studies, and an additional band at 24 kDa. The 14.8 and 24 kDa bands ...
Slide 1
... misfolded proteins as serious human diseases such as Alzheimers’s, Parkinson’s and mad cow disease coincide with the accumulation of misfolded proteins in the form of amyloids. • no consensus on how the amyloids and the disease itself is linked but it is becoming clear that a misfolded protein can b ...
... misfolded proteins as serious human diseases such as Alzheimers’s, Parkinson’s and mad cow disease coincide with the accumulation of misfolded proteins in the form of amyloids. • no consensus on how the amyloids and the disease itself is linked but it is becoming clear that a misfolded protein can b ...
Table S5. Proteins specifically induced or repressed during A
... (A) Genomic organization of JR1. Exons are indicated as rectangles. The triangle marks the region of T-DNA insertion. (B) qPCR analysis of JR1 expression in Col-0 or in the JR1 mutant line described in (A). Accumulation of the JR1 transcripts is expressed as fold change values related to the control ...
... (A) Genomic organization of JR1. Exons are indicated as rectangles. The triangle marks the region of T-DNA insertion. (B) qPCR analysis of JR1 expression in Col-0 or in the JR1 mutant line described in (A). Accumulation of the JR1 transcripts is expressed as fold change values related to the control ...
PowerPoint
... About (85) % of all plasma proteins are synthesized in the liver. The bulk of the remainder (particularly immunoglobulins) are synthesized by plasma cells and cells of reticuloendothelial system while the site of synthesis of most plasma proteins is known with some certainty; the site of degradation ...
... About (85) % of all plasma proteins are synthesized in the liver. The bulk of the remainder (particularly immunoglobulins) are synthesized by plasma cells and cells of reticuloendothelial system while the site of synthesis of most plasma proteins is known with some certainty; the site of degradation ...
Structural basis of ubiquitylation Andrew P VanDemark and
... powerful punch. Covalent attachment of the ubiquitin C terminus to substrate lysine residues, a process known as ubiquitylation, targets the substrate for a range of possible fates, the best known of which is degradation by the 26S proteasome, but which also include endocytosis, targeting to lysosom ...
... powerful punch. Covalent attachment of the ubiquitin C terminus to substrate lysine residues, a process known as ubiquitylation, targets the substrate for a range of possible fates, the best known of which is degradation by the 26S proteasome, but which also include endocytosis, targeting to lysosom ...
here
... Protein Diagram The diagram below shows a portion of a protein bound to a nucleotide structure. There are multiple interactions that bind the substrate to this active site. From the following choices correctly choose which answer correctly characterizes the shown interactions. A. B. C. D. E. F. ...
... Protein Diagram The diagram below shows a portion of a protein bound to a nucleotide structure. There are multiple interactions that bind the substrate to this active site. From the following choices correctly choose which answer correctly characterizes the shown interactions. A. B. C. D. E. F. ...
Proteins WORD 1000 KB - Science Learning Hub
... carbohydrates and fats, proteins are made up of the elements carbon (C), hydrogen (H) and (O) but they also contain nitrogen (N). Amino acids Proteins are very big molecules made up of smaller units known as ‘amino acids’. There are about 20 different naturally occurring amino acids that combine to ...
... carbohydrates and fats, proteins are made up of the elements carbon (C), hydrogen (H) and (O) but they also contain nitrogen (N). Amino acids Proteins are very big molecules made up of smaller units known as ‘amino acids’. There are about 20 different naturally occurring amino acids that combine to ...
Protein Kinases
... The reversible addition of phosphate groups to proteins is important for the transmission of signals within eukaryotic cells and, as a result, protein phosphorylation and dephosphorylation regulate many diverse cellular processes. As the number of known protein kinases has increased at an ever-accel ...
... The reversible addition of phosphate groups to proteins is important for the transmission of signals within eukaryotic cells and, as a result, protein phosphorylation and dephosphorylation regulate many diverse cellular processes. As the number of known protein kinases has increased at an ever-accel ...
midterm 2 asnwer scheme
... These groups are referred to as general acids or general bases The side chain of histidine often participates in concerted acid-base catalysis because its pKa range is close to physiological pH The protonated imidazole ring can serve as a general base Enzymes use several functional groups th ...
... These groups are referred to as general acids or general bases The side chain of histidine often participates in concerted acid-base catalysis because its pKa range is close to physiological pH The protonated imidazole ring can serve as a general base Enzymes use several functional groups th ...
L-‐Lysine Monohydrochloride [Feed Grade (78.8%)]
... animal by-‐product meals are blended with corn. In the past, excess protein was fed to meet the requirement of the first limiting amino acid. With the commercialization of L-‐Lysine, n ...
... animal by-‐product meals are blended with corn. In the past, excess protein was fed to meet the requirement of the first limiting amino acid. With the commercialization of L-‐Lysine, n ...
Chapter 33
... – A single folding pathway that has sequential transitional states that have limited flexibilities along the pathway or – Can have multiple transition states with similar energy values and a variety of pathways to get to the final ...
... – A single folding pathway that has sequential transitional states that have limited flexibilities along the pathway or – Can have multiple transition states with similar energy values and a variety of pathways to get to the final ...
File
... • Fibrous proteins, such as collagen, have structural functions. • Globular proteins, such as enzymes and haemoglobin, carry out metabolic functions. It is the very different structure and shape of each of these types of proteins that enables them to carry out their functions. ...
... • Fibrous proteins, such as collagen, have structural functions. • Globular proteins, such as enzymes and haemoglobin, carry out metabolic functions. It is the very different structure and shape of each of these types of proteins that enables them to carry out their functions. ...
Unit 3. Basic of Biopolymers (3) Control of Protein Function
... Iron binding regulates the repressor of the diphtheria toxin gene Comparison of the structures of the aporepressor DtxR (red, left, PDB 1dpr) and the ternary complex (right) of repressor (green), metal ion (Fe2+, orange) and DNA (grey) (PDB 1fst). Iron binding induces a conformational change that m ...
... Iron binding regulates the repressor of the diphtheria toxin gene Comparison of the structures of the aporepressor DtxR (red, left, PDB 1dpr) and the ternary complex (right) of repressor (green), metal ion (Fe2+, orange) and DNA (grey) (PDB 1fst). Iron binding induces a conformational change that m ...
Proteins
... Factors that must be supplied in the diet for the body to be able to synthesis PROTEİN include : 1 . all E.a.a consume simultaneously and in proper amount 2 . an adequate total amount of protein to supply amine groups to synthesis non – E.a.a 3 . adequate of CHO & FAT to spare protein being used to ...
... Factors that must be supplied in the diet for the body to be able to synthesis PROTEİN include : 1 . all E.a.a consume simultaneously and in proper amount 2 . an adequate total amount of protein to supply amine groups to synthesis non – E.a.a 3 . adequate of CHO & FAT to spare protein being used to ...
UBIQUITIN AT FOX CHASE
... 1982, Art Haas used isotope exchange at equilibrium to establish the reaction sequence and a number of equilibrium and rate constants of E1, the only enzyme of the cell that uses Ubiquitin, per se, as a substrate. The Ub activation process is defined by the formation of two equivalents of PPi, one e ...
... 1982, Art Haas used isotope exchange at equilibrium to establish the reaction sequence and a number of equilibrium and rate constants of E1, the only enzyme of the cell that uses Ubiquitin, per se, as a substrate. The Ub activation process is defined by the formation of two equivalents of PPi, one e ...
tutorial4_scoringMatices
... Why is BLOSUM62 called BLOSUM62? Basically, this is because all blocks whose members shared at least 62% identity with ANY other member of that block were averaged and represented as 1 sequence. ...
... Why is BLOSUM62 called BLOSUM62? Basically, this is because all blocks whose members shared at least 62% identity with ANY other member of that block were averaged and represented as 1 sequence. ...
Ubiquitin

Ubiquitin is a small (8.5 kDa) regulatory protein that has been found in almost all tissues (ubiquitously) of eukaryotic organisms. It was discovered in 1975 by Goldstein and further characterized throughout the 1970s and 1980s. There are four genes in the human genome that produce ubiquitin: UBB, UBC, UBA52 and RPS27A.The addition of ubiquitin to a substrate protein is called ubiquitination or ubiquitylation. Ubiquitination can affect proteins in many ways: It can signal for their degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitination is carried out in three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade binds ubiquitin to lysine residues on the protein substrate via an isopeptide bond or to the amino group of the protein's N-terminus via a peptide bond.The protein modifications can be either a single ubiquitin protein (monoubiquitination) or a chain of ubiquitin (polyubiquitination). The ubiquitination bonds are always formed with one of the seven lysine residues from the ubiquitin molecule. These 'linking' lysines are represented by a ""K"" (which is the one-letter amino acid notation of lysine) and a number, referring to its position in the ubiquitin molecule. First, a ubiquitin molecule is bonded by its C-terminus to a specific lysine residue (e.g. K48, K29, K63,...) on the target protein. Poly-ubiquitination occurs when the C-terminus of another ubiquitin, will be linked again to a lysine residue (for example again K48 or K29) on the previously added ubiquitin molecule, forming a chain. This process repeats several times, leading to the addition of several ubiquitins. Only poly-ubiquitination on defined lysines, mostly on K48 and K29, is related to degradation with the proteasome (referred to as the ""molecular kiss of death""), while other polyubiquitinations (e.g. on K63, K11, K6) and monoubiquitinations may regulate processes such as endocytic trafficking, inflammation, translation and DNA repair.Lysine 48-linked chains have been much-studied. They are the forms of chains that signal proteins to the proteasome, which destroys and recycles proteins. This discovery won the Nobel Prize for chemistry in 2004.















![L-‐Lysine Monohydrochloride [Feed Grade (78.8%)]](http://s1.studyres.com/store/data/007857369_1-57c2188e57086807bb71bba81a3737e6-300x300.png)





