Conformational Memory of Single Photosynthetic Pigment
... consequence of the conformational heterogeneity, protein ensembles exist in a broad variety of structures, which manifests itself as a dramatic increase in dynamic heterogeneity reflecting the distribution of the associated barriers that separate the various structures. In order to elucidate informa ...
... consequence of the conformational heterogeneity, protein ensembles exist in a broad variety of structures, which manifests itself as a dramatic increase in dynamic heterogeneity reflecting the distribution of the associated barriers that separate the various structures. In order to elucidate informa ...
Protein Electrophoresis
... How does SDS-PAGE separate proteins? Proteins produce a unique challenge for electrophoresis because they have complex shapes and different charges, which affect how they migrate through the gel. In order to accurately separate proteins by molecular weight and not by shape or charge, the secondary s ...
... How does SDS-PAGE separate proteins? Proteins produce a unique challenge for electrophoresis because they have complex shapes and different charges, which affect how they migrate through the gel. In order to accurately separate proteins by molecular weight and not by shape or charge, the secondary s ...
Tertiary Structure
... 1). Secondary structures are arranged in a few common patterns - i.e, resulting in protein “families”. 2). Proteins fold to form the most stable structure. Stability arises from: formation of large number of intramolecular hydrogen bonds reduction in hydrophobic surface area from solvent ...
... 1). Secondary structures are arranged in a few common patterns - i.e, resulting in protein “families”. 2). Proteins fold to form the most stable structure. Stability arises from: formation of large number of intramolecular hydrogen bonds reduction in hydrophobic surface area from solvent ...
2-Protein structure
... Protein misfolding • Alzheimer’s disease: • β amyloid protein is a misfolded protein. • It forms fibrous deposits or plaques in the brains of Alzheimer’s patients. • Creutzfeldt-Jacob or prion disease: • Prion protein is present in normal brain tissue. • In diseased brains, the same protein is misf ...
... Protein misfolding • Alzheimer’s disease: • β amyloid protein is a misfolded protein. • It forms fibrous deposits or plaques in the brains of Alzheimer’s patients. • Creutzfeldt-Jacob or prion disease: • Prion protein is present in normal brain tissue. • In diseased brains, the same protein is misf ...
1st Prize: Alex Davison
... possible that micelle-like oligomers distort transmembrane ion channels, increasing membrane permeability which leads to a higher concentration of calcium ions inside the neuron, thereby inducing an ER stress response culminating in apoptosis31. An indirect impact of both soluble oligomers and large ...
... possible that micelle-like oligomers distort transmembrane ion channels, increasing membrane permeability which leads to a higher concentration of calcium ions inside the neuron, thereby inducing an ER stress response culminating in apoptosis31. An indirect impact of both soluble oligomers and large ...
HERBALIFE Protein Snacks
... Protein Bars Deluxe Protein Bar Deluxe is a protein bar smothered in milk chocolate and packed with vitamins. It contains 10 grams of protein to sustain your energy and satisfy your appetite, and contains also Vitamins E, B6, B12, Niacin, Thiamine and Riboflavin. There are zero trans fats, no artifi ...
... Protein Bars Deluxe Protein Bar Deluxe is a protein bar smothered in milk chocolate and packed with vitamins. It contains 10 grams of protein to sustain your energy and satisfy your appetite, and contains also Vitamins E, B6, B12, Niacin, Thiamine and Riboflavin. There are zero trans fats, no artifi ...
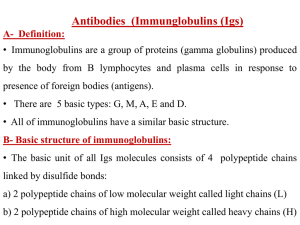
1. Inter-chain disulfide bonds
... the amino acid sequences in the heavy chains. Ig G a) IgG is the major Ig in serum:70-75% of serum Ig is IgG b) IgG is composed of one basic unit (monomer), i.e of low Mol. weight c) Placental transfer: IgG is the only class of Ig that crosses the placenta. In addition, Ig G is also transferred from ...
... the amino acid sequences in the heavy chains. Ig G a) IgG is the major Ig in serum:70-75% of serum Ig is IgG b) IgG is composed of one basic unit (monomer), i.e of low Mol. weight c) Placental transfer: IgG is the only class of Ig that crosses the placenta. In addition, Ig G is also transferred from ...
The UBA2 Domain Functions as an Intrinsic Stabilization Signal that
... The presence of several characteristic structural domains highlights the close relationship of Rad23 with the ubiquitin/proteasome system. Rad23 contains an N-terminal ubiquitin-like (UbL) domain and two ubiquitin-associated (UBA) domains: an internal UBA1 domain and a C-terminal UBA2 domain (Buchbe ...
... The presence of several characteristic structural domains highlights the close relationship of Rad23 with the ubiquitin/proteasome system. Rad23 contains an N-terminal ubiquitin-like (UbL) domain and two ubiquitin-associated (UBA) domains: an internal UBA1 domain and a C-terminal UBA2 domain (Buchbe ...
Proteins
... Protection of DNA Amplification of genetic information Efficient regulation of gene expression ...
... Protection of DNA Amplification of genetic information Efficient regulation of gene expression ...
Coarse Grained MD
... ENM reproduce very well the results of atomistic molecular dynamics. How can it work so well? Flexibility depends strongly on the shape and topology of the protein. Sequence has a minor effect in the global dynamics of the protein. Structure-based potentials can be only defined when the native confo ...
... ENM reproduce very well the results of atomistic molecular dynamics. How can it work so well? Flexibility depends strongly on the shape and topology of the protein. Sequence has a minor effect in the global dynamics of the protein. Structure-based potentials can be only defined when the native confo ...
Dynamical Analysis of Networks: How to Identify Important Nodes with
... ♦ Proteins function by binding to other molecules (ligands, proteins). ♦ Enzyme proteins catalyzes the chemical reactions by binding to reactant (substrate). ...
... ♦ Proteins function by binding to other molecules (ligands, proteins). ♦ Enzyme proteins catalyzes the chemical reactions by binding to reactant (substrate). ...
Amino acids in proteins
... Description of structure of proteins • the macromolecule contains various AAs, in an exactly defined order and quantity • spacial arrangement and biological function are DEPENDENT on the amino acid composition • native protein biological active conformation ...
... Description of structure of proteins • the macromolecule contains various AAs, in an exactly defined order and quantity • spacial arrangement and biological function are DEPENDENT on the amino acid composition • native protein biological active conformation ...
Proteins and Enzymes Assessment Statements 7.5.1 Explain the
... In competitive inhibition, a molecule, called a competitive inhibitor, competes directly for the active site of the enzyme. The result is that the substrate then has fewer encounters with the active site and the chemical reaction rate is decreased. The competitive inhibitor must have a similar struc ...
... In competitive inhibition, a molecule, called a competitive inhibitor, competes directly for the active site of the enzyme. The result is that the substrate then has fewer encounters with the active site and the chemical reaction rate is decreased. The competitive inhibitor must have a similar struc ...
WHAT IS PROTEIN?
... the essential amino acids our bodies cannot make and are therefore vital in our diets in small amounts. By contrast, incomplete proteins, which come from mainly plant sources, can be combined to make a complete protein. WHY IS PROTEIN IMPORTANT IN YOUR DIET? Protein makes up the largest percentage o ...
... the essential amino acids our bodies cannot make and are therefore vital in our diets in small amounts. By contrast, incomplete proteins, which come from mainly plant sources, can be combined to make a complete protein. WHY IS PROTEIN IMPORTANT IN YOUR DIET? Protein makes up the largest percentage o ...
C H E M I S T R Y
... • used to treat a disease that is caused by a gene that fails to produce a necessary protein or that produces a dysfunctional protein ...
... • used to treat a disease that is caused by a gene that fails to produce a necessary protein or that produces a dysfunctional protein ...
protease (NS34A) and an RNA polymerase
... fied ubiquitin alone could not fully activate parkin — complete activation required coin cident modification of parkin’s ubiquitin-like domain as well as of ubiquitin, each at their respective serine-65 residues. A unique aspect of this group’s work is their use of a strain of yeast that harbours a ...
... fied ubiquitin alone could not fully activate parkin — complete activation required coin cident modification of parkin’s ubiquitin-like domain as well as of ubiquitin, each at their respective serine-65 residues. A unique aspect of this group’s work is their use of a strain of yeast that harbours a ...
Basic Biochemistry - Personal Webspace for QMUL
... Smallest proteins fastest through the Strands Figure 3-9, page 73 (3-9, page 75) The protein separation provides a direct measurement of their ________ Proteins with known molecular masses are run as a scale marker beside those with unknown mass Differences in mass of ~2% between proteins ca ...
... Smallest proteins fastest through the Strands Figure 3-9, page 73 (3-9, page 75) The protein separation provides a direct measurement of their ________ Proteins with known molecular masses are run as a scale marker beside those with unknown mass Differences in mass of ~2% between proteins ca ...
ppt
... Glycosylation adds carbohydrate chains to proteins to form glycoproteins; occurs in ER and Golgi (Chapt. 10) • Carbohydrates: target proteins for transport to organelles, or secretion; recognition sites in cell-cell interactions. ...
... Glycosylation adds carbohydrate chains to proteins to form glycoproteins; occurs in ER and Golgi (Chapt. 10) • Carbohydrates: target proteins for transport to organelles, or secretion; recognition sites in cell-cell interactions. ...
Lecture2
... that targets proteins for rapid proteolysis. Ubiquitin is attached to the amino group of the side chain of a lysine residue, then more are added to form a chain. Polyubiquinated proteins are recognized and degraded by a large protease complex, the proteasome. Ubiquitin can have other functions: Ad ...
... that targets proteins for rapid proteolysis. Ubiquitin is attached to the amino group of the side chain of a lysine residue, then more are added to form a chain. Polyubiquinated proteins are recognized and degraded by a large protease complex, the proteasome. Ubiquitin can have other functions: Ad ...
Proteomics studies of post-translational modifications in plants
... from the Arabidopsis thylakoid membranes by trypsin and methylated on acidic residues to improve specific binding of phosphopeptides to IMAC. The enriched phosphopeptides were then sequenced using ESI MS/MS (Hansson and Vener, 2003). In addition to the five known phosphorylation sites in PSII protei ...
... from the Arabidopsis thylakoid membranes by trypsin and methylated on acidic residues to improve specific binding of phosphopeptides to IMAC. The enriched phosphopeptides were then sequenced using ESI MS/MS (Hansson and Vener, 2003). In addition to the five known phosphorylation sites in PSII protei ...
Poster - Protein Information Resource
... over many years. When to use UniProt Use UniProt to retrieve curated, reliable, comprehensive information on proteins. ...
... over many years. When to use UniProt Use UniProt to retrieve curated, reliable, comprehensive information on proteins. ...
SCI 241 Protein Article research wk 5 version 6 Protein and the
... SCI 241 Protein Article research wk 5 version 6 Protein and the Different Types ...
... SCI 241 Protein Article research wk 5 version 6 Protein and the Different Types ...
Protein Basics - Mid Atlantic Dairy Association
... • The Recommended Dietary Allowances (RDA) for protein are based on body weight and include age-related adjustments for the extra protein needed for growth. • A good rule of thumb is about ½ gram per pound of body weight for growing kids: ages 1-14 years for girls, and ages 1-18 for boys. • G ...
... • The Recommended Dietary Allowances (RDA) for protein are based on body weight and include age-related adjustments for the extra protein needed for growth. • A good rule of thumb is about ½ gram per pound of body weight for growing kids: ages 1-14 years for girls, and ages 1-18 for boys. • G ...
Ubiquitin

Ubiquitin is a small (8.5 kDa) regulatory protein that has been found in almost all tissues (ubiquitously) of eukaryotic organisms. It was discovered in 1975 by Goldstein and further characterized throughout the 1970s and 1980s. There are four genes in the human genome that produce ubiquitin: UBB, UBC, UBA52 and RPS27A.The addition of ubiquitin to a substrate protein is called ubiquitination or ubiquitylation. Ubiquitination can affect proteins in many ways: It can signal for their degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitination is carried out in three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade binds ubiquitin to lysine residues on the protein substrate via an isopeptide bond or to the amino group of the protein's N-terminus via a peptide bond.The protein modifications can be either a single ubiquitin protein (monoubiquitination) or a chain of ubiquitin (polyubiquitination). The ubiquitination bonds are always formed with one of the seven lysine residues from the ubiquitin molecule. These 'linking' lysines are represented by a ""K"" (which is the one-letter amino acid notation of lysine) and a number, referring to its position in the ubiquitin molecule. First, a ubiquitin molecule is bonded by its C-terminus to a specific lysine residue (e.g. K48, K29, K63,...) on the target protein. Poly-ubiquitination occurs when the C-terminus of another ubiquitin, will be linked again to a lysine residue (for example again K48 or K29) on the previously added ubiquitin molecule, forming a chain. This process repeats several times, leading to the addition of several ubiquitins. Only poly-ubiquitination on defined lysines, mostly on K48 and K29, is related to degradation with the proteasome (referred to as the ""molecular kiss of death""), while other polyubiquitinations (e.g. on K63, K11, K6) and monoubiquitinations may regulate processes such as endocytic trafficking, inflammation, translation and DNA repair.Lysine 48-linked chains have been much-studied. They are the forms of chains that signal proteins to the proteasome, which destroys and recycles proteins. This discovery won the Nobel Prize for chemistry in 2004.