Matter Vocab Part 4
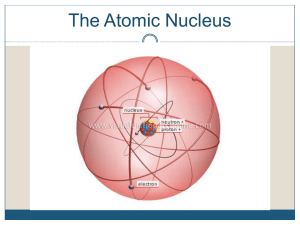
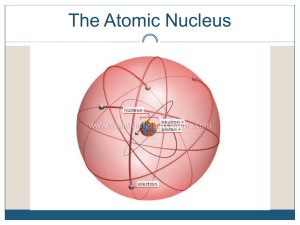
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
... Particle in the nucleus of an atom with a positive charge Particle in the nucleus of an atom with no charge Particle orbiting the nucleus of an atom with a negative charge Dense, central core of an atom (made of protons and neutrons) Path an electron takes around the nucleus A shell is sometimes cal ...
radioactivity-ppt
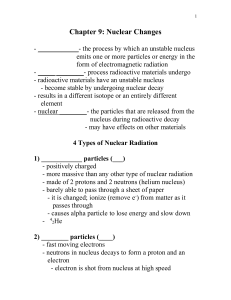
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
Physics 228 Today: April 22, 2012 Ch. 43 Nuclear
... Studies of nuclei have revealed that the protons and neutrons, are strongly attracted to each other, with the result that they are packed densely into a nucleus. It is a semi-reasonable approximation for nuclei to consider them to be spherical, and made up of dense hard-packed spheres, protons and n ...
... Studies of nuclei have revealed that the protons and neutrons, are strongly attracted to each other, with the result that they are packed densely into a nucleus. It is a semi-reasonable approximation for nuclei to consider them to be spherical, and made up of dense hard-packed spheres, protons and n ...
PHY140Y 33 Nuclear Properties - University of Toronto, Particle
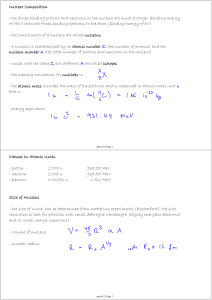
... A nucleus is characterized by the number of protons and neutrons it has. The number of protons, the “atomic number” or N , serves to define the type of nucleus it is and what element it is associated with, whereas the total number of protons and neutrons, the “atomic mass number” or A, is a measure o ...
... A nucleus is characterized by the number of protons and neutrons it has. The number of protons, the “atomic number” or N , serves to define the type of nucleus it is and what element it is associated with, whereas the total number of protons and neutrons, the “atomic mass number” or A, is a measure o ...
6.2 Atomic Nucleus Stability and Isotopes
... positron generated by decay is quickly annihilated by the relative excess of electrons. ...
... positron generated by decay is quickly annihilated by the relative excess of electrons. ...
Chapter 29: Nuclear Physics
... The absorbed dose of ionizing radiation is the amount of radiation energy absorbed per unit mass of tissue. Ionizing radiation is radiation with enough energy to ionize an atom or molecule. The SI unit of absorbed dose is the Gray. 1 Gy = 1 J/kg. Another common unit is the rad (radiation absorbed do ...
... The absorbed dose of ionizing radiation is the amount of radiation energy absorbed per unit mass of tissue. Ionizing radiation is radiation with enough energy to ionize an atom or molecule. The SI unit of absorbed dose is the Gray. 1 Gy = 1 J/kg. Another common unit is the rad (radiation absorbed do ...
Masses in Atomic Units - proton 1.007 u 938.28 MeV
... masses of the individual neutrons and protons making up the nucleus ...
... masses of the individual neutrons and protons making up the nucleus ...
2005 Nuclear FRQs - AP Chemistry Olympics
... Please include your name, school, school phone, name of principal/headmaster and school website address. Don’t forget to include the file format you want, Mac or PC. ...
... Please include your name, school, school phone, name of principal/headmaster and school website address. Don’t forget to include the file format you want, Mac or PC. ...
FUSION AND FISSION
... 500 million metric tons of hydrogen to helium. Due to the process of fusion, 5 million metric tons of excess material is converted into energy in each second. This means that every year, 157,680,000,000,000 metric tons are converted into energy. ...
... 500 million metric tons of hydrogen to helium. Due to the process of fusion, 5 million metric tons of excess material is converted into energy in each second. This means that every year, 157,680,000,000,000 metric tons are converted into energy. ...
Summative Assessment Review!
... • An alpha particle is simply a helium nuclei (He) which is ejected with high energy from an unstable nucleus ...
... • An alpha particle is simply a helium nuclei (He) which is ejected with high energy from an unstable nucleus ...
Chemistry: The Nature of Matter
... Elements ____________________________________________________________ ____________________________________________________________ Periodic Table of Elements Over 100 elements known, but only about 2 dozen commonly found in living systems ...
... Elements ____________________________________________________________ ____________________________________________________________ Periodic Table of Elements Over 100 elements known, but only about 2 dozen commonly found in living systems ...