Pure Substances and Mixtures
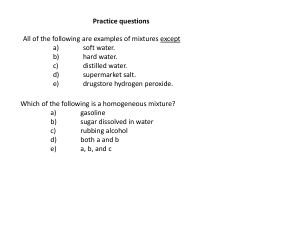
... – Elements are pure substances that cannot be broken down into simpler substances by ordinary chemical means. ...
... – Elements are pure substances that cannot be broken down into simpler substances by ordinary chemical means. ...
Introduction to Chemistry for Coach Keith`s Biology
... The K energy level is closest to the nucleus & only holds 2 electrons, while the L – Q energy levels can hold 8 electrons ...
... The K energy level is closest to the nucleus & only holds 2 electrons, while the L – Q energy levels can hold 8 electrons ...
GY 111 Lecture Note Series Elemental Chemistry
... chalcopyrite is CuFeS2 (one part copper + 1 part iron + 2 parts sulfur) Many of the man-made elements and even some of the naturally occurring ones are unstable. They tend to break apart over time through a process called or radioactivity. This must mean that there is something smaller than an atom ...
... chalcopyrite is CuFeS2 (one part copper + 1 part iron + 2 parts sulfur) Many of the man-made elements and even some of the naturally occurring ones are unstable. They tend to break apart over time through a process called or radioactivity. This must mean that there is something smaller than an atom ...
Nuclear Chemistry - Ector County ISD.
... Traditional chemical reactions occur as a result of the interaction between valence electrons around an atom's nucleus . In 1896, Henri Becquerel expanded the field of chemistry to include nuclear changes when he discovered that uranium emitted radiation. Soon after Becquerel's discovery, Marie Sklo ...
... Traditional chemical reactions occur as a result of the interaction between valence electrons around an atom's nucleus . In 1896, Henri Becquerel expanded the field of chemistry to include nuclear changes when he discovered that uranium emitted radiation. Soon after Becquerel's discovery, Marie Sklo ...
Prior knowledge catch-up student sheet for Chapter 3 Quantitative
... For example, the atomic number of sodium is 11 and the mass number is 23. Number of protons = 11 Number of electrons = 11 Number of neutrons = 23 − 11 = 12 Chemical reactions can be represented using a formula to show reactants and products in a chemical equation, with an arrow in between. An equati ...
... For example, the atomic number of sodium is 11 and the mass number is 23. Number of protons = 11 Number of electrons = 11 Number of neutrons = 23 − 11 = 12 Chemical reactions can be represented using a formula to show reactants and products in a chemical equation, with an arrow in between. An equati ...
on Nuclear Physics - Good Earth School
... Radioactive decay (also known as nuclear decay or radioactivity) is the process by which the nucleus of an unstable atom loses energy by emitting radiations ...
... Radioactive decay (also known as nuclear decay or radioactivity) is the process by which the nucleus of an unstable atom loses energy by emitting radiations ...
nuclear physics - rct study guide
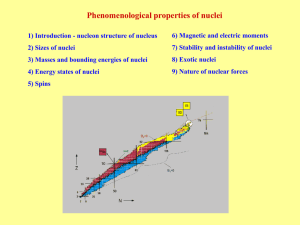
... On the average, approximately 200 MeV of energy is released per fission. The fission process can be "energetically" explained by comparing the critical energy for fission with the amount of energy input, i.e., the neutron binding energy. For U-238 and Th-232, the critical energy for fission is great ...
... On the average, approximately 200 MeV of energy is released per fission. The fission process can be "energetically" explained by comparing the critical energy for fission with the amount of energy input, i.e., the neutron binding energy. For U-238 and Th-232, the critical energy for fission is great ...
Particles and Waves Answers
... a) (ii) Individual electrons in the metal atom absorb the energy of a single photon, the energy of which is dependent on the frequency of the photon. The photon energy can be calculated using the equation E = hf. Below a certain frequency the energy absorbed by an electron in the metal, from the pho ...
... a) (ii) Individual electrons in the metal atom absorb the energy of a single photon, the energy of which is dependent on the frequency of the photon. The photon energy can be calculated using the equation E = hf. Below a certain frequency the energy absorbed by an electron in the metal, from the pho ...
Atomic Structure
... destroyed in ordinary chemical reactions. However, these changes CAN occur in nuclear reactions! Atoms of an element have a characteristic average mass which is unique to that element. Atoms of any one element differ in properties from atoms of another element ...
... destroyed in ordinary chemical reactions. However, these changes CAN occur in nuclear reactions! Atoms of an element have a characteristic average mass which is unique to that element. Atoms of any one element differ in properties from atoms of another element ...