Bioorganic chemistry-a scientific endeavour in continuous
... combinatory procedures; the polymerase chain reaction (PCR); all of the latest separation and spectroscopic methodology with computer analysis; and the generous use -as reagents -- of bacteria, fungi, enzymes, whole cells, and ground liver microsomes, inter alia. A graduate course in bioorganic chem ...
... combinatory procedures; the polymerase chain reaction (PCR); all of the latest separation and spectroscopic methodology with computer analysis; and the generous use -as reagents -- of bacteria, fungi, enzymes, whole cells, and ground liver microsomes, inter alia. A graduate course in bioorganic chem ...
Chapters 14
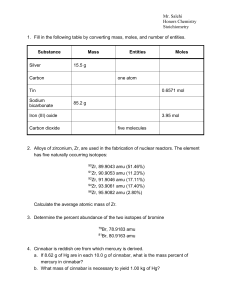
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
... 7. What is the molarity of a solution made by dissolving 9.1 g of H3PO4 in enough water to make 22.3 L of solution? Assume that H3PO4 ionizes completely in water to H+ and PO43ions. What is the pH of the solution? Find the concentration of OH-? ...
Spectroscopy In Oceanography
... sampling equipment. Although speculations regarding the saltiness of the sea were made by the ancient Greeks, systematic chemical measurements did not begin until the 18th and 19th Centuries. Robert Boyle (1670) and Lavoisier ( 1776) were among the early pioneers in identifying the various salts pre ...
... sampling equipment. Although speculations regarding the saltiness of the sea were made by the ancient Greeks, systematic chemical measurements did not begin until the 18th and 19th Centuries. Robert Boyle (1670) and Lavoisier ( 1776) were among the early pioneers in identifying the various salts pre ...
Chapter 2 - profpaz.com
... Atoms of the same element (same atomic number) can possess different number of neutrons (different mass numbers) and are called isotopes. Most elements have several isotopes, which are indicated by its chemical symbol, followed by a dash and the mass number of isotope. For example, the 3 isotopes of ...
... Atoms of the same element (same atomic number) can possess different number of neutrons (different mass numbers) and are called isotopes. Most elements have several isotopes, which are indicated by its chemical symbol, followed by a dash and the mass number of isotope. For example, the 3 isotopes of ...
Study of oxygen fugacity influence on redox state of iron in
... investigated: 1) granitic; 2) pantelleritic (alkali granitoid). Samples were melted in vertical muffle tube under controlled oxygen fugacity and then quenched in water. Alumina crucibles were used as a container for powdered rock samples. Microprobe analysis has shown that chemical composition chang ...
... investigated: 1) granitic; 2) pantelleritic (alkali granitoid). Samples were melted in vertical muffle tube under controlled oxygen fugacity and then quenched in water. Alumina crucibles were used as a container for powdered rock samples. Microprobe analysis has shown that chemical composition chang ...
Computers_in_chemistry - University of St Andrews
... What Kinds of Theoretical Chemistry can be Done? (1) Quantum Chemistry Density Functional Theory • Makes use of the theorem that all properties of interest can be determined directly from the electron density. • True in principle, but the correct “functional” is unknown. • Less rigorous than ab ini ...
... What Kinds of Theoretical Chemistry can be Done? (1) Quantum Chemistry Density Functional Theory • Makes use of the theorem that all properties of interest can be determined directly from the electron density. • True in principle, but the correct “functional” is unknown. • Less rigorous than ab ini ...
Stoichiometry - Cloudfront.net
... a. What is the mass % of each element in acetaminophen? b. How many grams of carbon are in a 1.41 g sample of acetaminophen? 6. A 2.074 g sample of an unknown compound was subjected to combustion analysis, and produced 3.800 g of CO2 and 1.040 g of H2O. Assuming the compound contains only carbon, hy ...
... a. What is the mass % of each element in acetaminophen? b. How many grams of carbon are in a 1.41 g sample of acetaminophen? 6. A 2.074 g sample of an unknown compound was subjected to combustion analysis, and produced 3.800 g of CO2 and 1.040 g of H2O. Assuming the compound contains only carbon, hy ...
35. Number of reactions - Royal Society of Chemistry
... (Cu(OH)2.CuCO3) is formed initially. On adding excess A this dissolves to form a deep blue solution of Cu(NH3)42+(aq). ...
... (Cu(OH)2.CuCO3) is formed initially. On adding excess A this dissolves to form a deep blue solution of Cu(NH3)42+(aq). ...
CHEM 401 Syllabus 2013
... cover course material presented since the previous exam. However, Physical Chemistry is comprehensive in nature, and understanding the previous topics is necessary to understand the current material. Additionally, on the last day of lecture, an assessment exam will be ...
... cover course material presented since the previous exam. However, Physical Chemistry is comprehensive in nature, and understanding the previous topics is necessary to understand the current material. Additionally, on the last day of lecture, an assessment exam will be ...
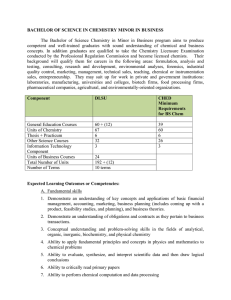
BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS
... BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS The Bachelor of Science Chemistry in Minor in Business program aims to produce competent and well-trained graduates with sound understanding of chemical and business concepts. In addition graduates are qualified to take the Chemistry Licensure Exami ...
... BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS The Bachelor of Science Chemistry in Minor in Business program aims to produce competent and well-trained graduates with sound understanding of chemical and business concepts. In addition graduates are qualified to take the Chemistry Licensure Exami ...
Job Description - Keele University
... and guidance on international qualifications at UK NARIC Visas and Nationality Please note this appointment is subject to medical clearance and on-going medical surveillance by the University Occupational Health provider. References You are asked to provide details of two referees (three if you are ...
... and guidance on international qualifications at UK NARIC Visas and Nationality Please note this appointment is subject to medical clearance and on-going medical surveillance by the University Occupational Health provider. References You are asked to provide details of two referees (three if you are ...
Chapter 1 Introduction: Matter and Measurement
... nature composed of 4 elements air, fire, water, and earth that have 4 properties – hotness, coldness, dryness, wetness (no atoms) 3. alchemists – furthered chemistry by trying to turn other metals into gold – discovered new compounds and elements 4. Robert Boyle -1661 rejected Greek concept of eleme ...
... nature composed of 4 elements air, fire, water, and earth that have 4 properties – hotness, coldness, dryness, wetness (no atoms) 3. alchemists – furthered chemistry by trying to turn other metals into gold – discovered new compounds and elements 4. Robert Boyle -1661 rejected Greek concept of eleme ...
S3 Chemistry - eduBuzz.org
... Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion. Calculate the charge on a ion using nuclide notation Use the periodic table to identify whether an element is a metal or non-meta ...
... Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion. Calculate the charge on a ion using nuclide notation Use the periodic table to identify whether an element is a metal or non-meta ...
Learning Outcomes for Chemical Reactions and
... • Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. • State the charge of an ion. • Calculate the charge on a ion using nuclide notation • Use the periodic table to identify whether an element is a metal or non-meta ...
... • Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. • State the charge of an ion. • Calculate the charge on a ion using nuclide notation • Use the periodic table to identify whether an element is a metal or non-meta ...
Introduction to Computational Chemistry
... flexibility and power of electronic computers, basic principles of classical and quantum mechanics are now implemented in a form which can handle the many-body problems associated with the structure and behavior of complex molecular systems." John A. Pople (November 1997) (Nobel prize for chemistry ...
... flexibility and power of electronic computers, basic principles of classical and quantum mechanics are now implemented in a form which can handle the many-body problems associated with the structure and behavior of complex molecular systems." John A. Pople (November 1997) (Nobel prize for chemistry ...
Title
... medicine. Paracelsus: „The true use of chemistry is not to make gold but to prepare medicines.” ...
... medicine. Paracelsus: „The true use of chemistry is not to make gold but to prepare medicines.” ...
Computers in Chemistry - University of St Andrews
... What Kinds of Theoretical Chemistry can be Done? (1) Quantum Chemistry Density Functional Theory • Makes use of the theorem that all properties of interest can be determined directly from the electron density. • True in principle, but the correct “functional” is unknown. • Less rigorous than ab ini ...
... What Kinds of Theoretical Chemistry can be Done? (1) Quantum Chemistry Density Functional Theory • Makes use of the theorem that all properties of interest can be determined directly from the electron density. • True in principle, but the correct “functional” is unknown. • Less rigorous than ab ini ...
ICP Plasma
... DC plasma relies on the gas between the two electrodes to have a potential high enough to be ionized. ...
... DC plasma relies on the gas between the two electrodes to have a potential high enough to be ionized. ...
EKSIKA JOINT EVALUATION TEST. Kenya Certificate
... If 6.8g of hydrogen peroxide contained 75cm3 of solution with water were completely decomposed, determine the rise in temperature due to the reaction.(Specific heat capacity of water =4.2Jg-1K-1 , density of water = 1g/cm3 , O = 16 , H = 1). ...
... If 6.8g of hydrogen peroxide contained 75cm3 of solution with water were completely decomposed, determine the rise in temperature due to the reaction.(Specific heat capacity of water =4.2Jg-1K-1 , density of water = 1g/cm3 , O = 16 , H = 1). ...
Chemical Reactions and The Mole Review
... • Do we need a break? http://www.youtube.com/watch?v=rWCvymhpfY&feature=channel&list=UL ...
... • Do we need a break? http://www.youtube.com/watch?v=rWCvymhpfY&feature=channel&list=UL ...
SAMPLE QUESTION PAPER-II Chemistry (Theory) Class-XII
... Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly and prepared azo dye successfully. (i) ...
... Due to holiday, they start preparing azodye but it cannot be prepared. Then their friend Reena told them to prepare benzene diazonium chloride again and to use it immediately to prepare azo dye and they proceed accordingly and prepared azo dye successfully. (i) ...
S3 Chemistry - eduBuzz.org
... Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion. Calculate the charge on a ion using nuclide notation Use the periodic table to identify whether an element is a metal or non-meta ...
... Identify whether a species has an equal or unequal number of protons and electrons and use this to state whether it is an atom or ion. State the charge of an ion. Calculate the charge on a ion using nuclide notation Use the periodic table to identify whether an element is a metal or non-meta ...
Analytical chemistry

Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample, and quantitative analysis determines the amount of certain components in the substance. The separation of components is often performed prior to analysis.Analytical methods can be separated into classical and instrumental. Classical methods (also known as wet chemistry methods) use separations such as precipitation, extraction, and distillation and qualitative analysis by color, odor, or melting point. Classical quantitative analysis is achieved by measurement of weight or volume. Instrumental methods use an apparatus to measure physical quantities of the analyte such as light absorption, fluorescence, or conductivity. The separation of materials is accomplished using chromatography, electrophoresis or field flow fractionation methods.Analytical chemistry is also focused on improvements in experimental design, chemometrics, and the creation of new measurement tools to provide better chemical information. Analytical chemistry has applications in forensics, bioanalysis, clinical analysis, environmental analysis, and materials analysis.