Spatial Distribution and Biodiversity of Kingdom Animalia at the
... meaning dominant species were different according to stations or seasons. BPI values for mammals and fish were high at four stations, meaning dominant species were recorded in those habitats. The values of ß-diversity for animals were varied from 0.229 for reptiles/amphibians to 0.269 for mammals (F ...
... meaning dominant species were different according to stations or seasons. BPI values for mammals and fish were high at four stations, meaning dominant species were recorded in those habitats. The values of ß-diversity for animals were varied from 0.229 for reptiles/amphibians to 0.269 for mammals (F ...
The Living Planet
... water molecule has “dissociated” into H+ and OH- ions. Now, of course, these oppositely charged ions are attracted to one another and they will re-associate. Water molecules are constantly splitting and rejoining in solution. In pure water, 1 in 10,000,000 (10 million) water molecules is dissociated ...
... water molecule has “dissociated” into H+ and OH- ions. Now, of course, these oppositely charged ions are attracted to one another and they will re-associate. Water molecules are constantly splitting and rejoining in solution. In pure water, 1 in 10,000,000 (10 million) water molecules is dissociated ...
Name ______ Period ______ 7th Grade Science Study Guide 1 7
... 2. Gaillard studies the effect of time spent practicing basketball on number of points scored in a game. 3. Kiki ask the question, “How does hours spent studying affect science grades?” 4. Peter wants to know how the amount of baking soda added to vinegar affects the amount of bubbles produced. 5. S ...
... 2. Gaillard studies the effect of time spent practicing basketball on number of points scored in a game. 3. Kiki ask the question, “How does hours spent studying affect science grades?” 4. Peter wants to know how the amount of baking soda added to vinegar affects the amount of bubbles produced. 5. S ...
File
... 4. The formula for sulfurous acid is. a. SF2 b. H2SO4 c. HSO3 d. HSF 5. Which of the following bonds is most negatively charged? a. C-H b. C-S c.C-N d. C-O 6. Based on symmetry which one of these molecules is non-polar? a. H3O+ b. PCl5 c. H2O d. NH3 7. The molecular shape of PH3 would be. a. Trigona ...
... 4. The formula for sulfurous acid is. a. SF2 b. H2SO4 c. HSO3 d. HSF 5. Which of the following bonds is most negatively charged? a. C-H b. C-S c.C-N d. C-O 6. Based on symmetry which one of these molecules is non-polar? a. H3O+ b. PCl5 c. H2O d. NH3 7. The molecular shape of PH3 would be. a. Trigona ...
Compound Name
... b) The forecast for next week is for a heat wave, smog and high humidity. c) When the film An Inconvenient Truth was released, 1998 was the hottest year on record. d) Vancouver generally experiences mild, wet winters due to its proximity to the Pacific Ocean. ...
... b) The forecast for next week is for a heat wave, smog and high humidity. c) When the film An Inconvenient Truth was released, 1998 was the hottest year on record. d) Vancouver generally experiences mild, wet winters due to its proximity to the Pacific Ocean. ...
Sustainable Food Production
... orchards, vineyards, vegetable and rice production and pasture for dairy cows and sheep. Australia has the highest water storage system in the world, where 70% is used for irrigation. To support the irrigation systems, the natural landscape is changed , dams and channels are built and paddocks are l ...
... orchards, vineyards, vegetable and rice production and pasture for dairy cows and sheep. Australia has the highest water storage system in the world, where 70% is used for irrigation. To support the irrigation systems, the natural landscape is changed , dams and channels are built and paddocks are l ...
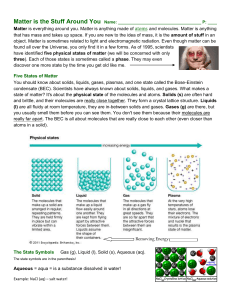
Stuff Matters Handout
... Elements and compounds can move from one physical state to another and not change their basic atomic parts. Oxygen (O2) as a gas still has the same properties as liquid oxygen. The liquid state is colder and denser, but the molecules (the basic parts) are still the same. Water (H2O) is another examp ...
... Elements and compounds can move from one physical state to another and not change their basic atomic parts. Oxygen (O2) as a gas still has the same properties as liquid oxygen. The liquid state is colder and denser, but the molecules (the basic parts) are still the same. Water (H2O) is another examp ...
Water Resources
... Consist of covalently bonded carbon atoms and often include other elements, especially hydrogen • ____________________: Organic compounds, such as petroleum, that contain only hydrogen and carbon • ____________________: Lack carbon-to-carbon bonds Organic compounds include natural gas, petroleum, co ...
... Consist of covalently bonded carbon atoms and often include other elements, especially hydrogen • ____________________: Organic compounds, such as petroleum, that contain only hydrogen and carbon • ____________________: Lack carbon-to-carbon bonds Organic compounds include natural gas, petroleum, co ...
Integrating Forest and Water Monitoring: A Pilot Study
... Forest Health Monitoring (FHM) plots in the DRB. ...
... Forest Health Monitoring (FHM) plots in the DRB. ...
7.2 Writing Chemical Equations
... substances change in his atomic theory. In a chemical reaction the ways in which the atoms are joined together are changed. As reactants are changed into products, bonds that hold atoms together are broken and new bonds are ...
... substances change in his atomic theory. In a chemical reaction the ways in which the atoms are joined together are changed. As reactants are changed into products, bonds that hold atoms together are broken and new bonds are ...
Chapter 6
... The law of conservation of mass states that: in a chemical reaction, the total mass of the reactants is always equal to the total mass of the products. Experiments show that atoms in a chemical reaction are not changed themselves, and the number of atoms has to stay the same from before the reactio ...
... The law of conservation of mass states that: in a chemical reaction, the total mass of the reactants is always equal to the total mass of the products. Experiments show that atoms in a chemical reaction are not changed themselves, and the number of atoms has to stay the same from before the reactio ...
$doc.title
... substance that can be weighed on an analytical balance. One molar mass of a substance contains Avogadro’s number of particles (6.02 x 1023), and would be described as one mole. For instance, the GFW of water, H2O, is 18.016 ((2 x 1.008) + 16.00 = 18.016). One mole of water would therefore have a mas ...
... substance that can be weighed on an analytical balance. One molar mass of a substance contains Avogadro’s number of particles (6.02 x 1023), and would be described as one mole. For instance, the GFW of water, H2O, is 18.016 ((2 x 1.008) + 16.00 = 18.016). One mole of water would therefore have a mas ...
Semester 2 Review WS
... Which diagrams show only molecules? ___________________________________________ How are these pictures different from one another? ...
... Which diagrams show only molecules? ___________________________________________ How are these pictures different from one another? ...
Reactive Materials - NC State University
... The reactivity of inorganic compounds may be frequently correlated with their “family” in the periodic table. Within a given family, similar types of behavior are observed with changes in the magnitude of reactivity varying consistently with atomic weight. The reactivity of chemical compounds shows ...
... The reactivity of inorganic compounds may be frequently correlated with their “family” in the periodic table. Within a given family, similar types of behavior are observed with changes in the magnitude of reactivity varying consistently with atomic weight. The reactivity of chemical compounds shows ...
H 2 SO 4
... • CO2 + H2O H+ + HCO3Pollutant SO2 is more acidic than CO2 Strong acid H2SO4, HNO3, and HCl are responsible for damaging acid rain ...
... • CO2 + H2O H+ + HCO3Pollutant SO2 is more acidic than CO2 Strong acid H2SO4, HNO3, and HCl are responsible for damaging acid rain ...
Specific Reactions Quiz.wpd
... a) various carbon products created due to lack of oxygen including solid carbon (black component) b) as air contacts the random carbon products (smaller hydrocarbons) created, they may further combust c) since energy is still tied up in carbon product bonds, energy is not released all at once d) the ...
... a) various carbon products created due to lack of oxygen including solid carbon (black component) b) as air contacts the random carbon products (smaller hydrocarbons) created, they may further combust c) since energy is still tied up in carbon product bonds, energy is not released all at once d) the ...
Unit 2
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
Unit 2
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
Activity 2 Modelling Convection Currents
... • For an open response activity, have research teams design their own models to demonstrate convection currents. Be prepared to discuss modifications or other ideas. You might provide students with a list of materials, for example, some of those listed for this activity: the beaker, glass tube, Bun ...
... • For an open response activity, have research teams design their own models to demonstrate convection currents. Be prepared to discuss modifications or other ideas. You might provide students with a list of materials, for example, some of those listed for this activity: the beaker, glass tube, Bun ...
Chapter #2-Newest CPub
... • Law of Mass Conservation: The total mass of substances does not change during a chemical reaction (Lavoisier). • Law of Definite (or Constant) Composition: No matter what its source, a particular chemical compound is composed of the same elements in the same parts (fractions) by mass (Proust). • T ...
... • Law of Mass Conservation: The total mass of substances does not change during a chemical reaction (Lavoisier). • Law of Definite (or Constant) Composition: No matter what its source, a particular chemical compound is composed of the same elements in the same parts (fractions) by mass (Proust). • T ...
CHAPTER 1 Practice Exercises 1.1 x = 12.3 g Cd 1.3 2.24845 ×12 u
... atomic numbers and the mass numbers vary with the number of neutrons in the atom, which does not affect the chemistry of the elements as much as the number of protons. ...
... atomic numbers and the mass numbers vary with the number of neutrons in the atom, which does not affect the chemistry of the elements as much as the number of protons. ...
station 1 earth`s layers
... Structure of the Earth System Station 1 – Earth’s Layers At this station, you will learn about the layers of the Earth. There are multiple layers of the Earth. The Earth layers are: the crust, the mantle, the outer core, and the inner core. Some of the layers are considered to parts of the lithosphe ...
... Structure of the Earth System Station 1 – Earth’s Layers At this station, you will learn about the layers of the Earth. There are multiple layers of the Earth. The Earth layers are: the crust, the mantle, the outer core, and the inner core. Some of the layers are considered to parts of the lithosphe ...
1/21/16, 11:23 AM
... of challenges to come, however, tempers this recognition. In order for Target 7.C to be met, “a further 1.1 billion people will need to gain access by 2015.”4 Though water scarcity is the result of varied factors, at the forefront is climate change leading to desertification. Climate change is a hot ...
... of challenges to come, however, tempers this recognition. In order for Target 7.C to be met, “a further 1.1 billion people will need to gain access by 2015.”4 Though water scarcity is the result of varied factors, at the forefront is climate change leading to desertification. Climate change is a hot ...