1. Name of substance and manufacturer/supplier: 1.1 Trade name
... Ham’s F-12 with 10 mg/l phenol red, with 1.176 g/l NaHCO3, with stable glutamine Cat.No.: FG 0815 ...
... Ham’s F-12 with 10 mg/l phenol red, with 1.176 g/l NaHCO3, with stable glutamine Cat.No.: FG 0815 ...
Exam Review
... 16. According to the octet rule, how many pairs of outer electrons do the most stable atoms have? 17. In the lanthanide series, as the atomic number increases, to which sublevel are electrons being added? 18. Give examples of molecules with the following shapes: a) linear b) trigonal planer c) bent ...
... 16. According to the octet rule, how many pairs of outer electrons do the most stable atoms have? 17. In the lanthanide series, as the atomic number increases, to which sublevel are electrons being added? 18. Give examples of molecules with the following shapes: a) linear b) trigonal planer c) bent ...
UNIT 2 – Chemical Quantities
... Ex1. A 1.000g sample of a pure compound containing only C and H was combusted. 0.6919g of water and 3.338g of carbon dioxide were produced. a. Calculate the masses of C and H in the sample. b. Find the empirical formula of the compound. ...
... Ex1. A 1.000g sample of a pure compound containing only C and H was combusted. 0.6919g of water and 3.338g of carbon dioxide were produced. a. Calculate the masses of C and H in the sample. b. Find the empirical formula of the compound. ...
semester two review sheet
... 5. What are the special properties of water and why do they occur? 6. Explain why solid ice is less than liquid water with regard to particle arrangement. 7. Why does a substance like sugar dissolve in water, but oil does not? SOLUTIONS 1. Define the following terms: solution, solvent, solute, dilut ...
... 5. What are the special properties of water and why do they occur? 6. Explain why solid ice is less than liquid water with regard to particle arrangement. 7. Why does a substance like sugar dissolve in water, but oil does not? SOLUTIONS 1. Define the following terms: solution, solvent, solute, dilut ...
Unit 1: Building Blocks Homework
... Which of the following particles contains a different number of electrons from the others? (You may wish to use page 1 of the data booklet to help you.) A B C D ...
... Which of the following particles contains a different number of electrons from the others? (You may wish to use page 1 of the data booklet to help you.) A B C D ...
Chapter 11 Chemical Reactions
... can even tell whether or not a single replacement reaction will happen: –Because some chemicals are more “active” than others –More active replaces less active ...
... can even tell whether or not a single replacement reaction will happen: –Because some chemicals are more “active” than others –More active replaces less active ...
Chemical Reactions
... We can even tell whether or not a single replacement reaction will happen: –Because some chemicals are more “active” than others –More active replaces less active There is a list on page 333 - called the ...
... We can even tell whether or not a single replacement reaction will happen: –Because some chemicals are more “active” than others –More active replaces less active There is a list on page 333 - called the ...
Please use your NUMERICAL RESPONSE SHEET to answer the
... Use the following diagram to answer then next 4 questions Zarley was studying the periodic table and looking for any patterns in the arrangement of the elements. ...
... Use the following diagram to answer then next 4 questions Zarley was studying the periodic table and looking for any patterns in the arrangement of the elements. ...
marking scheme
... in number reaching activation energy (increase in number being effective) / only the high energy collisions lead to reaction (are effective) / leads to more (increase in) effective collisions / more collisions reach activation energy / number of collisions reaching (exceeding) activation energy crit ...
... in number reaching activation energy (increase in number being effective) / only the high energy collisions lead to reaction (are effective) / leads to more (increase in) effective collisions / more collisions reach activation energy / number of collisions reaching (exceeding) activation energy crit ...
Distribution and ecology of soldier fly larvae captured in Flemish
... in the water. Each aquatic habitat was explored in order to collect the highest possible richness of macroinvertebrates. For this purpose, kick sampling was performed. In addition to handnet sampling, animals were manually picked from stones, leaves and branches. Conductivity, dissolved oxygen and p ...
... in the water. Each aquatic habitat was explored in order to collect the highest possible richness of macroinvertebrates. For this purpose, kick sampling was performed. In addition to handnet sampling, animals were manually picked from stones, leaves and branches. Conductivity, dissolved oxygen and p ...
Chemistry! - Duplin County Schools
... • A chemical equation shows what happens during a chemical reaction • There is a reactant, an arrow, and a product in every chemical equation (RAP) • It is important for you to know if chemical equations are balanced or not ...
... • A chemical equation shows what happens during a chemical reaction • There is a reactant, an arrow, and a product in every chemical equation (RAP) • It is important for you to know if chemical equations are balanced or not ...
Classification of
... e) ______heterogeneous_________________ - mixture with individual parts visible f) _______states of matter_______________________ - solid, liquid, gas g) ___________solid___________________ - definite volume and shape h) ____________liquid__________________ - definite volume, changeable shape i) ___ ...
... e) ______heterogeneous_________________ - mixture with individual parts visible f) _______states of matter_______________________ - solid, liquid, gas g) ___________solid___________________ - definite volume and shape h) ____________liquid__________________ - definite volume, changeable shape i) ___ ...
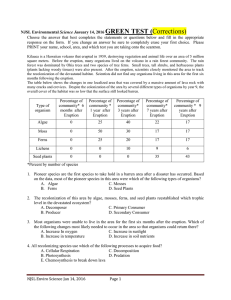
Environmental Science Exams and Keys Corrected 2016 Season
... 45. A population is a group of individuals of the same species. Can the proportion of individuals with certain traits in a population change because the environment changes? A. Yes, when the environment changes, individuals in a population can change their inherited traits to better fit the environ ...
... 45. A population is a group of individuals of the same species. Can the proportion of individuals with certain traits in a population change because the environment changes? A. Yes, when the environment changes, individuals in a population can change their inherited traits to better fit the environ ...
Water Management Statement
... facilities are located to mitigate the physical, regulatory and social risks that affect our longterm access to quality freshwater. We have undertaken a preliminary risk assessment of our operational supply chain using WWF’s Water Risk Filter to know which facilities and geographies are most at risk ...
... facilities are located to mitigate the physical, regulatory and social risks that affect our longterm access to quality freshwater. We have undertaken a preliminary risk assessment of our operational supply chain using WWF’s Water Risk Filter to know which facilities and geographies are most at risk ...
Green Living
... from the Inside Think about where your food comes from, remember you are what you eat. Buy organic whenever possible Grown in soils without chemical fertilizers, not sprayed with herbicides or pesticides, not processed with high heat and preservative chemicals to extend the shelf life WWII c ...
... from the Inside Think about where your food comes from, remember you are what you eat. Buy organic whenever possible Grown in soils without chemical fertilizers, not sprayed with herbicides or pesticides, not processed with high heat and preservative chemicals to extend the shelf life WWII c ...
File
... 55. Which pair of solutions forms a buffer when equal volumes of each are mixed? A) 0.20 M HCl and 0.20 M NaCl C) 0.20 M HCl and 0.20 M NH3 B) 0.40 M HC2H3O2 and 0.20 M NaOH D) 0.40 M HCl and 0.20 M NH3 56. A student is attempting to standardize a NaOH solution with a 0.500 molar solution of oxalic ...
... 55. Which pair of solutions forms a buffer when equal volumes of each are mixed? A) 0.20 M HCl and 0.20 M NaCl C) 0.20 M HCl and 0.20 M NH3 B) 0.40 M HC2H3O2 and 0.20 M NaOH D) 0.40 M HCl and 0.20 M NH3 56. A student is attempting to standardize a NaOH solution with a 0.500 molar solution of oxalic ...
QA1
... sulphur and iodine sublimes. (b) Nitrates(III) nitrates(V) except those of potassium, sodium and ammonium give brown fumes of nitrogen dioxide. (c) Many carbonates, all hydrogen carbonates, ethanoates (these also give carbon monoxide). (d) Sulphate(IV) (except those of sodium and potassium), thiosul ...
... sulphur and iodine sublimes. (b) Nitrates(III) nitrates(V) except those of potassium, sodium and ammonium give brown fumes of nitrogen dioxide. (c) Many carbonates, all hydrogen carbonates, ethanoates (these also give carbon monoxide). (d) Sulphate(IV) (except those of sodium and potassium), thiosul ...
Document
... The combination of 2 or more substances to form a compound Only one product General form: element or compound + element or compound compound ...
... The combination of 2 or more substances to form a compound Only one product General form: element or compound + element or compound compound ...
Show - Evonik
... This GPS Safety Summary is based on Evonik´s present knowledge and experience as of the date of issue. However, it implies no liability or other legal responsibility on the part of Evonik, including with regards to existing third party intellectual property rights, especially patent rights. In no ev ...
... This GPS Safety Summary is based on Evonik´s present knowledge and experience as of the date of issue. However, it implies no liability or other legal responsibility on the part of Evonik, including with regards to existing third party intellectual property rights, especially patent rights. In no ev ...
- Palisades School District
... The conjugate base of a weak acid reacts with water (hydrolysis) to reform the acid. Likewise, the conjugate acid of a weak base reacts with water to reform the base. ...
... The conjugate base of a weak acid reacts with water (hydrolysis) to reform the acid. Likewise, the conjugate acid of a weak base reacts with water to reform the base. ...