Question Bank - Edudel.nic.in
... Define water of crystallisation. Write the number of water molecules present in one formula unit of copper sulphate crystal. ...
... Define water of crystallisation. Write the number of water molecules present in one formula unit of copper sulphate crystal. ...
Threatened pond endemicity on an oceanic island: the presence of
... sampled the abundance of H. guernei using a protocol that targeted macroinvertebrates in three temporary and two permanent ponds on Terceira Island, Azores archipelago. We also recorded the abundance of G. holbrooki, which was observed for the first time on the island, as well as the environmental c ...
... sampled the abundance of H. guernei using a protocol that targeted macroinvertebrates in three temporary and two permanent ponds on Terceira Island, Azores archipelago. We also recorded the abundance of G. holbrooki, which was observed for the first time on the island, as well as the environmental c ...
Mechanisms and energetics of surface reactions at the copper
... Even if the initial oxygen coverage would be very low (as can be achieved experimentally by mechanical polishing and chemical reduction (Clendening and Campbell 1989)), the oxide layer could grow also in anoxic water due to the cleavage of water molecules. By cleaving the water molecules in S1, ini ...
... Even if the initial oxygen coverage would be very low (as can be achieved experimentally by mechanical polishing and chemical reduction (Clendening and Campbell 1989)), the oxide layer could grow also in anoxic water due to the cleavage of water molecules. By cleaving the water molecules in S1, ini ...
materials required/recommended for this paper
... The Haber process is the final step in the production of ammonia. It involves the reaction of nitrogen and hydrogen gases in the presence of an iron/iron oxide catalyst. This process is carried out at 350-550 C and 15-35 MPa. The reaction can be represented by the equation below. ...
... The Haber process is the final step in the production of ammonia. It involves the reaction of nitrogen and hydrogen gases in the presence of an iron/iron oxide catalyst. This process is carried out at 350-550 C and 15-35 MPa. The reaction can be represented by the equation below. ...
Chemistry MCQs - Target Publications
... ___________ law of combining volumes states that “Whenever gases combine, they do so in simple ratio by volumes”. (A) Avogadro’s (B) Gay Lussac’s (C) Dalton’s (D) Thomson’s The sum of the masses of reactants and products is equal in any physical or chemical reaction. This is in accordance with (A) L ...
... ___________ law of combining volumes states that “Whenever gases combine, they do so in simple ratio by volumes”. (A) Avogadro’s (B) Gay Lussac’s (C) Dalton’s (D) Thomson’s The sum of the masses of reactants and products is equal in any physical or chemical reaction. This is in accordance with (A) L ...
Reactions of Plutonium Dioxide with Water and Oxygen
... (approximately 0.1 g) were accurately weighed prior to placement in the Pt sample container. The oxide was outgassed to constant mass in vacuum at 400eC and was then exposed to water pressure at 15 tom as the temperature was increased stepwise from 25°C to 50, 100, 150, and 250°C over a period in ex ...
... (approximately 0.1 g) were accurately weighed prior to placement in the Pt sample container. The oxide was outgassed to constant mass in vacuum at 400eC and was then exposed to water pressure at 15 tom as the temperature was increased stepwise from 25°C to 50, 100, 150, and 250°C over a period in ex ...
ХИМИЯ НА АНГЛИЙСКОМ ЯЗЫКЕ
... 3.15. When 10.24 g of Cu is heated in an atmosphere of oxygen, 11.52 g of an oxide of copper is produced. What is the empirical formula of the oxide formed? 3.16. A 2.522 g sample of pure caffeine contains 1.248 g of carbon, 0.130 g of hydrogen, 0.728 g of nitrogen and 0.416 g of oxygen. What is the ...
... 3.15. When 10.24 g of Cu is heated in an atmosphere of oxygen, 11.52 g of an oxide of copper is produced. What is the empirical formula of the oxide formed? 3.16. A 2.522 g sample of pure caffeine contains 1.248 g of carbon, 0.130 g of hydrogen, 0.728 g of nitrogen and 0.416 g of oxygen. What is the ...
File
... Limiting Reactant • In the real world, reactants are not present in the exact mole ratio described by the balanced equation. • This means that one of the reactants will be used up before the other one. – The limiting reactant is used up first and restricts (stops) the reaction – The excess reactant ...
... Limiting Reactant • In the real world, reactants are not present in the exact mole ratio described by the balanced equation. • This means that one of the reactants will be used up before the other one. – The limiting reactant is used up first and restricts (stops) the reaction – The excess reactant ...
Magic of Chemical Reactions 2. - mt
... this, air and moisture cannot come in contact with the iron object and hence rusting doesn’t takes place. So, grills of doors and windows are always painted before they are used. *2. Physical states of reactants and products are mentioned while writing a chemical equation. Ans. 1. The equation that ...
... this, air and moisture cannot come in contact with the iron object and hence rusting doesn’t takes place. So, grills of doors and windows are always painted before they are used. *2. Physical states of reactants and products are mentioned while writing a chemical equation. Ans. 1. The equation that ...
Determination of Cystein and Methionine by Oscillating Chemical
... 3.1. Influence of experimental variables on the oscillating conductance In order to ensure the maximum possible sensitivity and precision in the determination as well as the ability to perform large series of analyses, working conditions were optimized considering three factors, namely: (i) accompli ...
... 3.1. Influence of experimental variables on the oscillating conductance In order to ensure the maximum possible sensitivity and precision in the determination as well as the ability to perform large series of analyses, working conditions were optimized considering three factors, namely: (i) accompli ...
Properties of Systems in Equilibrium - Le
... 5. Continue to add the 0.3 M HCl solution to the Pb(NO3)2 solution in the large test tube in roughly 1 mL increments until you just begin to see white PbCl2 solid appear in your test tube. To confirm that the solid is present, let the test tube sit on the bench for about 3 minutes, allowing all soli ...
... 5. Continue to add the 0.3 M HCl solution to the Pb(NO3)2 solution in the large test tube in roughly 1 mL increments until you just begin to see white PbCl2 solid appear in your test tube. To confirm that the solid is present, let the test tube sit on the bench for about 3 minutes, allowing all soli ...
Basic Agricultural Chemistry - Macmillan Education South Africa
... one another so strongly that they move quite far apart and as a result the cohesive forces between them are extremely weak. A liquid has a distinct volume independent of its container, but has no specific shape. It assumes the shape of the portion of the container that it occupies. The particles in ...
... one another so strongly that they move quite far apart and as a result the cohesive forces between them are extremely weak. A liquid has a distinct volume independent of its container, but has no specific shape. It assumes the shape of the portion of the container that it occupies. The particles in ...
Equilibrium - District 196
... reactants, products or neither are favored? • If Keq > 1, the forward reaction (products) are favored • If Keq < 1, the reverse reaction (reactants) are favored • If K = 1, neither reaction is favored (the concentrations of both products and reactants should be equal) ...
... reactants, products or neither are favored? • If Keq > 1, the forward reaction (products) are favored • If Keq < 1, the reverse reaction (reactants) are favored • If K = 1, neither reaction is favored (the concentrations of both products and reactants should be equal) ...
The Logical Structure of Organic Chemistry and the Empirical
... to cause wave functions or atomic orbitals to be taken as concrete entities, though they are nothing but the basis sets with which wave functions are expanded. In other words, a set of orbitals counts as a form of coordinate system, and therefore, as sometimes pointed out, assignment of electrons to ...
... to cause wave functions or atomic orbitals to be taken as concrete entities, though they are nothing but the basis sets with which wave functions are expanded. In other words, a set of orbitals counts as a form of coordinate system, and therefore, as sometimes pointed out, assignment of electrons to ...
Unit 3 Homework Booklet
... Two chemicals A and B react in solution to form C. The reaction has an activation energy of 150 kJ mol-1. If hydrogen ions are used as a catalyst the activation energy is 50 kJ mol-1. The enthalpy change for the reaction is -125 kJ mol-1. Present this information as a potential energy diagram using ...
... Two chemicals A and B react in solution to form C. The reaction has an activation energy of 150 kJ mol-1. If hydrogen ions are used as a catalyst the activation energy is 50 kJ mol-1. The enthalpy change for the reaction is -125 kJ mol-1. Present this information as a potential energy diagram using ...
Document
... with a high rate. Some reactions take hundreds, maybe even thousands, of years while others can happen in less than one second. If you want to think of a very slow reaction, think about how long it takes plants and ancient fish to become fossils (carbonization). Ultimately: Molecules moving too slow ...
... with a high rate. Some reactions take hundreds, maybe even thousands, of years while others can happen in less than one second. If you want to think of a very slow reaction, think about how long it takes plants and ancient fish to become fossils (carbonization). Ultimately: Molecules moving too slow ...
Structural characterisation of groundwater hydrophobic
... Elemental data for SRFA were supplied by the IHSS with the sample. Representative samples were collected on 12th October 1996 (3 · 10 L) and 18th March 2002 (150 L). These samples highlight the structural changes observed over the study period (1996–2003) and data for other samples are not included ...
... Elemental data for SRFA were supplied by the IHSS with the sample. Representative samples were collected on 12th October 1996 (3 · 10 L) and 18th March 2002 (150 L). These samples highlight the structural changes observed over the study period (1996–2003) and data for other samples are not included ...
LESSON 23: Exploding Bags
... between the atoms are broken or formed. During a chemical reaction, the structure or composition of the materials changes. Chemical reactions occur around us all the time. They even take place inside of our bodies. When we breathe, we take in oxygen from the air, which combines with glucose (a sugar ...
... between the atoms are broken or formed. During a chemical reaction, the structure or composition of the materials changes. Chemical reactions occur around us all the time. They even take place inside of our bodies. When we breathe, we take in oxygen from the air, which combines with glucose (a sugar ...
Swedish Marine Sciences Conference
... as abundance declines? (ii) Do teleost fishes possess greater intrinsic recovery abilities (as reflected by rmax) than other vertebrates? (iii) Does magnitude of population reduction influence the probability of recovery? (iv) If fish evolve in response to fishing, changing average fitness and thus ...
... as abundance declines? (ii) Do teleost fishes possess greater intrinsic recovery abilities (as reflected by rmax) than other vertebrates? (iii) Does magnitude of population reduction influence the probability of recovery? (iv) If fish evolve in response to fishing, changing average fitness and thus ...
ii. year course contents
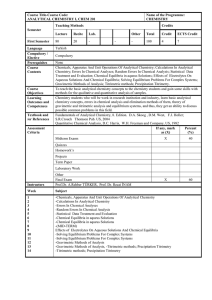
... To teach the basic analytical chemistry concepts to the chemistry students and gain some skills with methods for the qualitative and quantitative analysis of samples. Chemistry students who will be work in research institution and industry, learn basic analytical chemistry concepts, errors in chemic ...
... To teach the basic analytical chemistry concepts to the chemistry students and gain some skills with methods for the qualitative and quantitative analysis of samples. Chemistry students who will be work in research institution and industry, learn basic analytical chemistry concepts, errors in chemic ...
The Chemistry of Aqueous Systems
... P1 V1 P2 V2 (4.42 atm) (1 L) V1 X 4.42 L of air 1atm Solution: First, set up Boyle's Law equation: P1 equals 1 atm; V1 equals "X", P2 equals 65 psi and V2 equals 1 L. Remember, though, that you may not mix units. To bypass this fiasco, convert the 65 psi into atm (4.42 atm). Rearrange the eq ...
... P1 V1 P2 V2 (4.42 atm) (1 L) V1 X 4.42 L of air 1atm Solution: First, set up Boyle's Law equation: P1 equals 1 atm; V1 equals "X", P2 equals 65 psi and V2 equals 1 L. Remember, though, that you may not mix units. To bypass this fiasco, convert the 65 psi into atm (4.42 atm). Rearrange the eq ...
Chemical Reaction
... Metals are usually solid, shiny and strong. They are also good conductors of heat and electricity. Metals can be changed into new substances when they are involved in a chemical reaction. Some metals can react with acids. This type of chemical reaction is called corrosion. ...
... Metals are usually solid, shiny and strong. They are also good conductors of heat and electricity. Metals can be changed into new substances when they are involved in a chemical reaction. Some metals can react with acids. This type of chemical reaction is called corrosion. ...
CHEM181H1_06_2013_Y_P1
... This paper consists of 16 pages including the cover page, periodic table and two data sheets. Please ensure that you have them all. The use of non-programmable electronic calculators is permitted. ANSWER ALL QUESTIONS DIRECTLY ON THE PAPER AND WHERE NECESSARY OVER THE PAGE. Examiner ...
... This paper consists of 16 pages including the cover page, periodic table and two data sheets. Please ensure that you have them all. The use of non-programmable electronic calculators is permitted. ANSWER ALL QUESTIONS DIRECTLY ON THE PAPER AND WHERE NECESSARY OVER THE PAGE. Examiner ...
Determination and validation of an aquatic Maximum
... uncertainty of the risk assessment attributable to interspecies differences in sensitivity. In addition, this approach may reduce the uncertainty resulting from differences in the sensitivity of standard test species and those expected to be exposed in nature by also using non-standard test species ...
... uncertainty of the risk assessment attributable to interspecies differences in sensitivity. In addition, this approach may reduce the uncertainty resulting from differences in the sensitivity of standard test species and those expected to be exposed in nature by also using non-standard test species ...
Chem expo 12
... eat, the chemicals and fertilisers used to grow this food, the fuels we use for transport and energy and the wide range of medications that we use to prolong and enhance our lives all require thorough chemical analysis to ensure that they will perform their intended function and will not be harmful ...
... eat, the chemicals and fertilisers used to grow this food, the fuels we use for transport and energy and the wide range of medications that we use to prolong and enhance our lives all require thorough chemical analysis to ensure that they will perform their intended function and will not be harmful ...