Hydrogen peroxide solution about 30% w/v AnalaR
... If local regulations permit, mop up with plenty of water and run to waste, diluting greatly with running water. Otherwise absorb on an inert absorbent, transfer to container and arrange removal by disposal company. Ventilate area to dispel residual vapour. For large spillages liquids should be conta ...
... If local regulations permit, mop up with plenty of water and run to waste, diluting greatly with running water. Otherwise absorb on an inert absorbent, transfer to container and arrange removal by disposal company. Ventilate area to dispel residual vapour. For large spillages liquids should be conta ...
Constructive and Destructive Forces Study Guide
... A. GPS – helps seismologists measure movement of __faults_________ B. Seismographs – records movement of the Earth’s crust C. Beach restoration – replacing sand on the beach D. Jetty – prevents the current from carrying away ___sand________ E. Levee – keeps rising water within channels F. Coral reef ...
... A. GPS – helps seismologists measure movement of __faults_________ B. Seismographs – records movement of the Earth’s crust C. Beach restoration – replacing sand on the beach D. Jetty – prevents the current from carrying away ___sand________ E. Levee – keeps rising water within channels F. Coral reef ...
Biology Fall Semester Test 1 Study Guide
... The closing of its shell when a clam is removed from its watery environment is an example of how a clam maintains its: In a trophic pyramid, _______% of the energy from a source is passed on to the next level All living things are ________________, meaning they can’t survive on their own. The study ...
... The closing of its shell when a clam is removed from its watery environment is an example of how a clam maintains its: In a trophic pyramid, _______% of the energy from a source is passed on to the next level All living things are ________________, meaning they can’t survive on their own. The study ...
5 Themes of Geography
... a large, tall, rocky area of land that comes up out of the earth’s surface ...
... a large, tall, rocky area of land that comes up out of the earth’s surface ...
Model 2 – The Carbon Cycle
... what happens to the waste in nature? Why aren’t we up to our necks in natural refuse? Why is there always a supply of water? Why is there oxygen to breathe and carbon dioxide for photosynthesis? Organic compounds in nature are also recycled. This recycling process converts the complex organic compou ...
... what happens to the waste in nature? Why aren’t we up to our necks in natural refuse? Why is there always a supply of water? Why is there oxygen to breathe and carbon dioxide for photosynthesis? Organic compounds in nature are also recycled. This recycling process converts the complex organic compou ...
River Monitoring Plan for 2012 - South Yuba River Citizens League
... Dissolved Oxygen is the amount of oxygen dissolved in water and is measured in micrograms per liter (mg/L) or parts per million (ppm). River systems both consume and produce oxygen, and the vast majority of aquatic organisms require oxygen to survive, grow, and reproduce. Certain species require hig ...
... Dissolved Oxygen is the amount of oxygen dissolved in water and is measured in micrograms per liter (mg/L) or parts per million (ppm). River systems both consume and produce oxygen, and the vast majority of aquatic organisms require oxygen to survive, grow, and reproduce. Certain species require hig ...
Irreversible Changes
... Rusting and the burning of a fuel in the presence of oxygen are both chemical reactions known as oxidation. Iron, in the presence of water and oxygen, reacts to form iron oxide or rust. Whilst the rust can be rubbed off to reveal the ‘good’ iron underneath, the top layer has been changed and removed ...
... Rusting and the burning of a fuel in the presence of oxygen are both chemical reactions known as oxidation. Iron, in the presence of water and oxygen, reacts to form iron oxide or rust. Whilst the rust can be rubbed off to reveal the ‘good’ iron underneath, the top layer has been changed and removed ...
IPC Final Exam Review
... 3. Calculate the density of an object that has a mass of 237 grams and measures 5cm x 7cm x 3cm. ...
... 3. Calculate the density of an object that has a mass of 237 grams and measures 5cm x 7cm x 3cm. ...
Chapter 11: The rise of oxygen and ozone – ppt
... Detrital refers to minerals that were transported from their origin to the site of deposition in its original solid form, not in solution. Geologists can usually identify such rocks. For uranium, the more oxidized (U6+) is soluble, while the more reduced (U4+) is not soluble. Thus, the presence of d ...
... Detrital refers to minerals that were transported from their origin to the site of deposition in its original solid form, not in solution. Geologists can usually identify such rocks. For uranium, the more oxidized (U6+) is soluble, while the more reduced (U4+) is not soluble. Thus, the presence of d ...
Document
... • Reclamation of the sites shall be completed to restore the approved post mining land use according to the requirements of the original permit. ...
... • Reclamation of the sites shall be completed to restore the approved post mining land use according to the requirements of the original permit. ...
Chemistry Notes
... Separate the water in salt water from the salts Boil off the water and salts will remain Separate a mixture of gases Cool them – they will condense at different temperatures ...
... Separate the water in salt water from the salts Boil off the water and salts will remain Separate a mixture of gases Cool them – they will condense at different temperatures ...
Unit 2. EARTH`S RELIEF 1. THE EARTH
... • Mountains. They are elevations of the land with different origin. – They were created during the orogenies some millions years ago. – The highest mountains emerged in more recent periods. Their profiles are steeper. – The oldest mountains are lower and rounder. They are calle ...
... • Mountains. They are elevations of the land with different origin. – They were created during the orogenies some millions years ago. – The highest mountains emerged in more recent periods. Their profiles are steeper. – The oldest mountains are lower and rounder. They are calle ...
CHEM 301: AQUEOUS ENVIRONMENTAL CHEMISTRY
... Great Lks (NA) – contamination with toxic substances from industry (heavy metals, chemical waste and pesticides) curtailed in the 1970s and 80s. Concs ...
... Great Lks (NA) – contamination with toxic substances from industry (heavy metals, chemical waste and pesticides) curtailed in the 1970s and 80s. Concs ...
4 Chemistry
... Numbers that sit after and below the symbol. Represent number of atoms of that particular element. Numbers used to show how many molecules and/or compounds there are in a substance Are used to show reactions All substances needed for the reaction to take place All substances are on the left hand ...
... Numbers that sit after and below the symbol. Represent number of atoms of that particular element. Numbers used to show how many molecules and/or compounds there are in a substance Are used to show reactions All substances needed for the reaction to take place All substances are on the left hand ...
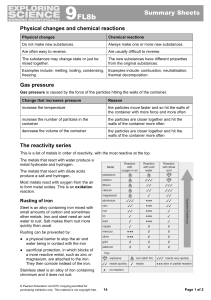
9F Reactivity - Parrs Wood High School
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
... These substances contain hydrogen and carbon only. They burn in a plentiful supply of air to form carbon dioxide and water: hydrocarbon + oxygen → carbon dioxide + water The test for oxygen is that it relights a glowing splint. An input of energy from a flame or spark is needed to start the combusti ...
Name__________________________ Period_______ Word
... much more information. Conditions under which a reaction occurs are often found above the arrow. An example of a reaction condition is the heat symbol (∆ ), which indicates that the reactants were heated. An equation can also indicate the physical state of the reactants and products. The physical st ...
... much more information. Conditions under which a reaction occurs are often found above the arrow. An example of a reaction condition is the heat symbol (∆ ), which indicates that the reactants were heated. An equation can also indicate the physical state of the reactants and products. The physical st ...
Non-Metals
... Nitrogen is notoriously inert . However it will combine with oxygen at high temperatures to form nitrous oxides . This occurs during lightning discharges and in the engines of vehicles . It will also combine with hydrogen under certain conditions of temperature and pressure to form ammonia . ...
... Nitrogen is notoriously inert . However it will combine with oxygen at high temperatures to form nitrous oxides . This occurs during lightning discharges and in the engines of vehicles . It will also combine with hydrogen under certain conditions of temperature and pressure to form ammonia . ...
Conclusion
... concentration of the Ca2+ and Mg2+ kations in hard water. These resources are available in different places of the world as this issue is worldwide and concerns every country. Most of them are found in nature but are also chemical. To soften water we can use Sodium bicarbonate NaHCO3 (baking soda), ...
... concentration of the Ca2+ and Mg2+ kations in hard water. These resources are available in different places of the world as this issue is worldwide and concerns every country. Most of them are found in nature but are also chemical. To soften water we can use Sodium bicarbonate NaHCO3 (baking soda), ...
Interdependence /53 1. Name the type of organism that is found at
... Algae blocks the light from reaching plants in the water. Plants can no longer photosynthesis and die. Bacteria break down the dead plants. The bacteria remove oxygen from the water for respiration. The oxygen levels decrease in the water so fish etc cannot respire, fish die. 11. What are ...
... Algae blocks the light from reaching plants in the water. Plants can no longer photosynthesis and die. Bacteria break down the dead plants. The bacteria remove oxygen from the water for respiration. The oxygen levels decrease in the water so fish etc cannot respire, fish die. 11. What are ...
Erosion, Transport, Deposition Key Words
... rock into fragments (rocks and stones) freeze-thaw action and rocks broken apart by plant roots. ...
... rock into fragments (rocks and stones) freeze-thaw action and rocks broken apart by plant roots. ...