Chapter 8: Chemical Reactions and Physical Changes
... • Mass number: total protons and neutrons in an atom’s nucleus • Atomic mass: the average mass of a sample of atoms of that element found in nature • Periodic table: chart that arranges elements by atomic number into rows and columns according to similarities in their properties ...
... • Mass number: total protons and neutrons in an atom’s nucleus • Atomic mass: the average mass of a sample of atoms of that element found in nature • Periodic table: chart that arranges elements by atomic number into rows and columns according to similarities in their properties ...
Atomic Structure Video Guide
... 12. If Carbon has 6 electrons then it has 6 _________________________. 13. Atomic Mass is the number of _______________________ and _____________________ in an atom. 14. Silicon (Si) is a major element that makes up ____________. It has ___________ Protons and _____________ Neutrons which makes up a ...
... 12. If Carbon has 6 electrons then it has 6 _________________________. 13. Atomic Mass is the number of _______________________ and _____________________ in an atom. 14. Silicon (Si) is a major element that makes up ____________. It has ___________ Protons and _____________ Neutrons which makes up a ...
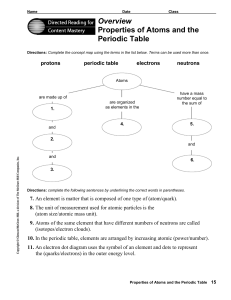
Overview Properties of Atoms and the Periodic Table
... . They will also have the same number of ...
... . They will also have the same number of ...
Outline Chapter 10 The Periodic Law
... • The alkaline earth metals are less active than the alkali metals. They are in group 2. • The inert gases are inactive nonmetals. They are in group 8. 10-7. The Periodic Table Formulated by Russian chemist Dmitri Mendeleev in ~1869 Periodic law=states that when elements are listed in order of atomi ...
... • The alkaline earth metals are less active than the alkali metals. They are in group 2. • The inert gases are inactive nonmetals. They are in group 8. 10-7. The Periodic Table Formulated by Russian chemist Dmitri Mendeleev in ~1869 Periodic law=states that when elements are listed in order of atomi ...
Unit 1 Test Study Guide KEY
... Neutron – found in the nucleus of the atom – no charge (neutral) Electron – found in the electron clouds outside the nucleus – negative charge 10. Compare elements, compounds, and mixtures. Element: consists of only one kind of atom, cannot be broken down into a simpler type of matter by either phys ...
... Neutron – found in the nucleus of the atom – no charge (neutral) Electron – found in the electron clouds outside the nucleus – negative charge 10. Compare elements, compounds, and mixtures. Element: consists of only one kind of atom, cannot be broken down into a simpler type of matter by either phys ...
The Periodic Table: Trends
... acknowledging its orderliness. There are exceptions to trends at times. To help you to focus on the trends, try to look first from peak to peak or from valley to valley. If the charts on the following pages were roller coaster rides, which elements are found at each of the highest points? Is there a ...
... acknowledging its orderliness. There are exceptions to trends at times. To help you to focus on the trends, try to look first from peak to peak or from valley to valley. If the charts on the following pages were roller coaster rides, which elements are found at each of the highest points? Is there a ...
Scientists timeline
... • English chemist and teacher • Pioneered the modern discovery process about atoms • Transformed Democritus’s theories on atoms into an actual scientific theory ...
... • English chemist and teacher • Pioneered the modern discovery process about atoms • Transformed Democritus’s theories on atoms into an actual scientific theory ...
Chapter 4
... He set it up in patterns. He predicted that some elements had not been discovered yet and left space for them on the table. No one believed him, until the elements were discovered and had the characteristics he said they would have. He set it up according to increasing atomic mass. Today it is by at ...
... He set it up in patterns. He predicted that some elements had not been discovered yet and left space for them on the table. No one believed him, until the elements were discovered and had the characteristics he said they would have. He set it up according to increasing atomic mass. Today it is by at ...
Topic 3 – Atoms and the Periodic Table – Learning Outcomes
... Group 8 Nobel Gases Between groups 2 and 3 we find the transition metals ...
... Group 8 Nobel Gases Between groups 2 and 3 we find the transition metals ...
Chapter 5/6 Notes
... Predicting properties using other elements data: Example: Predict the density of Aluminum given: Density: Ga = 5.9 g/cm3 & B = 2.3 g/cm3 ...
... Predicting properties using other elements data: Example: Predict the density of Aluminum given: Density: Ga = 5.9 g/cm3 & B = 2.3 g/cm3 ...
KWL chart and chem notes
... 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
... 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
Lecture 3
... s level for each shelf, three equivalent p levels for shelf 2 and above and 5 equivalent d levels for the third shelf and above. The difference between the three equivalent 2p levels is their arrangement in space. Otherwise they are equivalent in shape. Similarly for the d levels. ...
... s level for each shelf, three equivalent p levels for shelf 2 and above and 5 equivalent d levels for the third shelf and above. The difference between the three equivalent 2p levels is their arrangement in space. Otherwise they are equivalent in shape. Similarly for the d levels. ...
Periodic Table
... • When writing isotopes, the atomic number (or number of protons) will appear at the __________ • The mass number (number of protons plus neutrons will appear at the __________ • The element symbol will appear to the __________ • The different number of neutrons has NO bearing on chemical reactivity ...
... • When writing isotopes, the atomic number (or number of protons) will appear at the __________ • The mass number (number of protons plus neutrons will appear at the __________ • The element symbol will appear to the __________ • The different number of neutrons has NO bearing on chemical reactivity ...
Midterm Practice Test Answers
... 3. Write the isotope symbol, including atomic number and mass number for the following isotopes. (Isotope symbol ...
... 3. Write the isotope symbol, including atomic number and mass number for the following isotopes. (Isotope symbol ...
Bill Nye Atoms Workseet
... There are lots of _______________ _________________ in the rubbing alcohol where water molecules can fit. The main difference between aluminum and copper is the ___________________ of ___________________ in the nucleus. Scientists have grouped all the different elements into groups which have come t ...
... There are lots of _______________ _________________ in the rubbing alcohol where water molecules can fit. The main difference between aluminum and copper is the ___________________ of ___________________ in the nucleus. Scientists have grouped all the different elements into groups which have come t ...
Learning Objectives
... b. atomic number and mass number c. atomic weight and mass number 5. Explain how the atomic number and mass number of an atom can be used to determine the number of neutrons. 6. Explain how two isotopes of an element are similar. Explain how they are different. 7. Describe two biological application ...
... b. atomic number and mass number c. atomic weight and mass number 5. Explain how the atomic number and mass number of an atom can be used to determine the number of neutrons. 6. Explain how two isotopes of an element are similar. Explain how they are different. 7. Describe two biological application ...
are made up of
... that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron arrangement from your textbook.In Table I, write.the maximum number of electrons that can f ...
... that grouped elements accordingto their properties. They found that these properties repeated in a regular or periodic manner. This fact was used to predict properties of undiscovered elements. Reviewelectron arrangement from your textbook.In Table I, write.the maximum number of electrons that can f ...
Chapter 04
... What is the difference between atomic symbols and the way information is presented in the boxes on the periodic table? You should be able to use an atomic symbol to calculate the number of protons, neutrons, and electrons indicated by the symbol. You should also be able to use the number of protons, ...
... What is the difference between atomic symbols and the way information is presented in the boxes on the periodic table? You should be able to use an atomic symbol to calculate the number of protons, neutrons, and electrons indicated by the symbol. You should also be able to use the number of protons, ...
Chapter 04
... What is the difference between atomic symbols and the way information is presented in the boxes on the periodic table? You should be able to use an atomic symbol to calculate the number of protons, neutrons, and electrons indicated by the symbol. You should also be able to use the number of ...
... What is the difference between atomic symbols and the way information is presented in the boxes on the periodic table? You should be able to use an atomic symbol to calculate the number of protons, neutrons, and electrons indicated by the symbol. You should also be able to use the number of ...
Periodic table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. The table also shows four rectangular blocks: s-, p- d- and f-block. In general, within one row (period) the elements are metals on the lefthand side, and non-metals on the righthand side.The rows of the table are called periods; the columns are called groups. Six groups (columns) have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships between the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences.Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 94 elements exist naturally, although some are found only in trace amounts and were synthesized in laboratories before being found in nature. Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories. It has been shown that einsteinium and fermium once occurred in nature but currently do not. Synthesis of elements having higher atomic numbers is being pursued. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.