Chapter 10:The Mole
... The reduced ratio would be1:4:12 What about 1,000,000 atoms of each…. The reduced ratio would be1:4:12 Is there a number of atoms where the numbers 1:4:12 would be equal to 1g:4g:12g? ….YES…that number is the mole ...
... The reduced ratio would be1:4:12 What about 1,000,000 atoms of each…. The reduced ratio would be1:4:12 Is there a number of atoms where the numbers 1:4:12 would be equal to 1g:4g:12g? ….YES…that number is the mole ...
Lecture 11 Aerosol Generation and Measurements
... Since the AMS uses electron impact ionization and high temperature, species are modified as they are desorbed and ionized. Luckily, marker species and co-varying peaks can be found that uniquely identify compound classes. A high-resolution Time-Of-Flight Mass Spectrometer (TOFMS) has been developed ...
... Since the AMS uses electron impact ionization and high temperature, species are modified as they are desorbed and ionized. Luckily, marker species and co-varying peaks can be found that uniquely identify compound classes. A high-resolution Time-Of-Flight Mass Spectrometer (TOFMS) has been developed ...
May 1998
... M98T.1—Carnot Engine Problem A Carnot engine uses n moles of an ideal gas as its working substance. The absolute temperatures of its hot and cold reservoirs are denoted by T1 and T2 , respectively. The net work performed by the engine in one cycle of operation is W. The specific heats of the gas may ...
... M98T.1—Carnot Engine Problem A Carnot engine uses n moles of an ideal gas as its working substance. The absolute temperatures of its hot and cold reservoirs are denoted by T1 and T2 , respectively. The net work performed by the engine in one cycle of operation is W. The specific heats of the gas may ...
Chemistry Vocabulary List
... charge, nearly all of the mass, but a very small fraction of the volume of an atom. 2. Proton => Positively charged particle located in the nucleus of an atom; charge = +1, mass = 1 atomic mass unit 3. Neutron => Neutrally charged particle located in the nucleus of an atom; charge = 0, mass = 1 atom ...
... charge, nearly all of the mass, but a very small fraction of the volume of an atom. 2. Proton => Positively charged particle located in the nucleus of an atom; charge = +1, mass = 1 atomic mass unit 3. Neutron => Neutrally charged particle located in the nucleus of an atom; charge = 0, mass = 1 atom ...
January 2001
... Deduce the relation between the magnetic field Bz at radius R and the magnetic field Bz,ave averaged over the area of the circle. Also deduce the maximum energy E to which an electron could be accelerated by a betatron in terms of Bz , dBz,ave /dt and R. Hints: The electrons in this problem are ultr ...
... Deduce the relation between the magnetic field Bz at radius R and the magnetic field Bz,ave averaged over the area of the circle. Also deduce the maximum energy E to which an electron could be accelerated by a betatron in terms of Bz , dBz,ave /dt and R. Hints: The electrons in this problem are ultr ...
numerical evidence of the haldane conjecture
... in a local magnetic field (Wang, Lauwers-Rittenberg). After testing their performances, we chose the Wang method. Our algorithm satisfies the detailed balance property. The Fortuin-Kasteleyn clusters were created by using the HoshenKopelman procedure. The initial random vector was generated by ...
... in a local magnetic field (Wang, Lauwers-Rittenberg). After testing their performances, we chose the Wang method. Our algorithm satisfies the detailed balance property. The Fortuin-Kasteleyn clusters were created by using the HoshenKopelman procedure. The initial random vector was generated by ...
atomic theory student ch 4-10
... A. beginning of modern chemistry. B. his theory - back to Democritus' theory. ...
... A. beginning of modern chemistry. B. his theory - back to Democritus' theory. ...
Slides - Indico
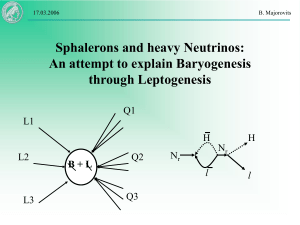
... It seems very difficult to explain Baryogenesis directly Extension of SM: Right handed neutrinos (Majorana Singlet) are required for consistency of the theory! They could also explain low ...
... It seems very difficult to explain Baryogenesis directly Extension of SM: Right handed neutrinos (Majorana Singlet) are required for consistency of the theory! They could also explain low ...
Principles of Technology
... In 1934, French physicists Frédéric Joliot-Curie and Irene Joliot-Curie bombarded aluminum-27 and produced the first artificially radioactive isotope, phosphorus-30: The phosphorus-30 undergoes positron decay (see pages 472—473) and forms silicon-30. 1. Which statement is false? a. Nuclear reactions ...
... In 1934, French physicists Frédéric Joliot-Curie and Irene Joliot-Curie bombarded aluminum-27 and produced the first artificially radioactive isotope, phosphorus-30: The phosphorus-30 undergoes positron decay (see pages 472—473) and forms silicon-30. 1. Which statement is false? a. Nuclear reactions ...
Assignment for Physics 295 – Professor Thomson – due May 2 2005
... detector. However, one electron and 4 jets of particles are seen in the detector with energy and momentum given by (Ee,pe), (E1,p1), (E2,p2), (E3,p3) and (E4,p4), where bold font indicates a vector. In this case, the top quark (q=+2/3) decayed into a bottom-quark (q=-1/3) and a W+ boson that subsequ ...
... detector. However, one electron and 4 jets of particles are seen in the detector with energy and momentum given by (Ee,pe), (E1,p1), (E2,p2), (E3,p3) and (E4,p4), where bold font indicates a vector. In this case, the top quark (q=+2/3) decayed into a bottom-quark (q=-1/3) and a W+ boson that subsequ ...
doc
... detector. However, one electron and 4 jets of particles are seen in the detector with energy and momentum given by (Ee,pe), (E1,p1), (E2,p2), (E3,p3) and (E4,p4), where bold font indicates a vector. In this case, the top quark (q=+2/3) decayed into a bottom-quark (q=-1/3) and a W+ boson that subsequ ...
... detector. However, one electron and 4 jets of particles are seen in the detector with energy and momentum given by (Ee,pe), (E1,p1), (E2,p2), (E3,p3) and (E4,p4), where bold font indicates a vector. In this case, the top quark (q=+2/3) decayed into a bottom-quark (q=-1/3) and a W+ boson that subsequ ...
Qu`attendre des premières données du LHC
... compared to predictions from ZEUS PDF AFTER these data are included in the fit ...
... compared to predictions from ZEUS PDF AFTER these data are included in the fit ...
Particle detectors - Teaching Advanced Physics
... the particle, and also to its lifetime if it decays with a very short half life. Magnetic fields deflect charged particles and so bend their tracks. The curvature depends on momentum, charge and the strength of the field. How could you tell whether a particle had positive or negative charge? (Curvin ...
... the particle, and also to its lifetime if it decays with a very short half life. Magnetic fields deflect charged particles and so bend their tracks. The curvature depends on momentum, charge and the strength of the field. How could you tell whether a particle had positive or negative charge? (Curvin ...
Atomic Structure Development
... Walther Nernst and the transition to modern physical science Niels Bohr and the quantum atom The Fly in the Cathedral The Elements Science in the 20th Century Nobel Prize Lecture ...
... Walther Nernst and the transition to modern physical science Niels Bohr and the quantum atom The Fly in the Cathedral The Elements Science in the 20th Century Nobel Prize Lecture ...
Structures Topic 4 PwrPt
... arranged in layers, but the forces between the layers are relatively weak. Because the layers slide over one another easily, graphite is slippery and makes a good dry lubricant. The layers of graphite ina pencil “lead” rub off and leave a mark on the paper when you write. ...
... arranged in layers, but the forces between the layers are relatively weak. Because the layers slide over one another easily, graphite is slippery and makes a good dry lubricant. The layers of graphite ina pencil “lead” rub off and leave a mark on the paper when you write. ...
Chapter 4: Old Models of the Atom
... In 1911, Rutherford took time out of his usual schedule of wresting tigers and making out with hot chicks to do some more experimenting with nuclear stuff. In this experiment, he shot a beam of positivelycharged alpha particles10 at a really thin piece of gold foil to see what would happen. He surro ...
... In 1911, Rutherford took time out of his usual schedule of wresting tigers and making out with hot chicks to do some more experimenting with nuclear stuff. In this experiment, he shot a beam of positivelycharged alpha particles10 at a really thin piece of gold foil to see what would happen. He surro ...
Physics_A2_36_ChargedParticlesInCircularOrbits
... A beam of electrons with a velocity of 3.2x107 m/s is fired into a uniform magnetic field which has a flux density of 8.5mT. The initial velocity is perpendicular to the field. Explain why the electrons move in a circular orbit Calculate the radius of the orbit What must the flux density be adjuste ...
... A beam of electrons with a velocity of 3.2x107 m/s is fired into a uniform magnetic field which has a flux density of 8.5mT. The initial velocity is perpendicular to the field. Explain why the electrons move in a circular orbit Calculate the radius of the orbit What must the flux density be adjuste ...
Topic 7: Atomic and nuclear physics 7.1 The atom
... • When ionizing radiation passes through the tube, some of the gas molecules are ionized, creating positively charged ions, and electrons. The strong electric field created by the tube's electrodes accelerates the ions towards the cathode and the electrons towards the anode. The ion pairs gain suffi ...
... • When ionizing radiation passes through the tube, some of the gas molecules are ionized, creating positively charged ions, and electrons. The strong electric field created by the tube's electrodes accelerates the ions towards the cathode and the electrons towards the anode. The ion pairs gain suffi ...
Standard Model
The Standard Model of particle physics is a theory concerning the electromagnetic, weak, and strong nuclear interactions, as well as classifying all the subatomic particles known. It was developed throughout the latter half of the 20th century, as a collaborative effort of scientists around the world. The current formulation was finalized in the mid-1970s upon experimental confirmation of the existence of quarks. Since then, discoveries of the top quark (1995), the tau neutrino (2000), and more recently the Higgs boson (2013), have given further credence to the Standard Model. Because of its success in explaining a wide variety of experimental results, the Standard Model is sometimes regarded as a ""theory of almost everything"".Although the Standard Model is believed to be theoretically self-consistent and has demonstrated huge and continued successes in providing experimental predictions, it does leave some phenomena unexplained and it falls short of being a complete theory of fundamental interactions. It does not incorporate the full theory of gravitation as described by general relativity, or account for the accelerating expansion of the universe (as possibly described by dark energy). The model does not contain any viable dark matter particle that possesses all of the required properties deduced from observational cosmology. It also does not incorporate neutrino oscillations (and their non-zero masses).The development of the Standard Model was driven by theoretical and experimental particle physicists alike. For theorists, the Standard Model is a paradigm of a quantum field theory, which exhibits a wide range of physics including spontaneous symmetry breaking, anomalies, non-perturbative behavior, etc. It is used as a basis for building more exotic models that incorporate hypothetical particles, extra dimensions, and elaborate symmetries (such as supersymmetry) in an attempt to explain experimental results at variance with the Standard Model, such as the existence of dark matter and neutrino oscillations.