CHAPTER 4 SOLUTION STOICHIOMETRY 1 CHAPTER FOUR
... a. Polarity is a term applied to covalent compounds. Polar covalent compounds have an unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges like in ...
... a. Polarity is a term applied to covalent compounds. Polar covalent compounds have an unequal sharing of electrons in bonds that results in unequal charge distribution in the overall molecule. Polar molecules have a partial negative end and a partial positive end. These are not full charges like in ...
On the composition of ammonia–sulfuric
... NH3 and H2 SO4 , and then grew to larger clusters containing more than 50 molecules of NH3 and H2 SO4 , corresponding to mobility-equivalent diameters greater than 2 nm. Water molecules evaporate from these clusters during sampling and are not observed. We found that the composition of the NH3 –H2 S ...
... NH3 and H2 SO4 , and then grew to larger clusters containing more than 50 molecules of NH3 and H2 SO4 , corresponding to mobility-equivalent diameters greater than 2 nm. Water molecules evaporate from these clusters during sampling and are not observed. We found that the composition of the NH3 –H2 S ...
Cr (VI) REMOVAL WITH NATURAL, SURFACTANT - Library
... the local clinoptilolite rich mineral and its surfactant modified forms and to investigate potential applications of clinoptilolite rich mineral, surfactant modified clinoptilolite rich mineral and bacteria loaded forms in Cr (VI) sorption. Characterizations of clinoptilolite rich mineral and its mo ...
... the local clinoptilolite rich mineral and its surfactant modified forms and to investigate potential applications of clinoptilolite rich mineral, surfactant modified clinoptilolite rich mineral and bacteria loaded forms in Cr (VI) sorption. Characterizations of clinoptilolite rich mineral and its mo ...
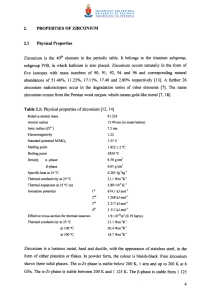
2. PROPERTIES OF ZIRCONIUM 2.1 Physical Properties
... Zirconium powder reacts with many other elements, such as hydrogen, boron, carbon, nitrogen and the halogens. The ignition temperature for the process is above 200°C. The reaction of platinum and zirconium powder is violent [12, 13, 15]. ...
... Zirconium powder reacts with many other elements, such as hydrogen, boron, carbon, nitrogen and the halogens. The ignition temperature for the process is above 200°C. The reaction of platinum and zirconium powder is violent [12, 13, 15]. ...
Amphiphilic Sugar Metal Carbenes
... toothpaste being well known examples.8 Low molecular mass gelators that form aggregates are investigated as counterparts to formally used polymers. These amphiphilic molecules form aggregates in a solvent through forces that are not covalent bonds like in polymers, which make them more interesting. ...
... toothpaste being well known examples.8 Low molecular mass gelators that form aggregates are investigated as counterparts to formally used polymers. These amphiphilic molecules form aggregates in a solvent through forces that are not covalent bonds like in polymers, which make them more interesting. ...
Soot Formation Modeling during Hydrocarbon
... developed. The models were validated by means of a suitable numerical technique (discrete Galerkin method). The gas-phase chemistry of soot precursor and particle formation was described in terms of different pathways. Accordingly, the formation and evolution of soot particles differs with respect t ...
... developed. The models were validated by means of a suitable numerical technique (discrete Galerkin method). The gas-phase chemistry of soot precursor and particle formation was described in terms of different pathways. Accordingly, the formation and evolution of soot particles differs with respect t ...
Handbook of Inorganic Chemicals Pradyot Patnaik, Ph.D.
... substances are soluble in water and other polar solvents while the non-polar, covalent compounds are more soluble in the non-polar solvents. In sparingly soluble, slightly soluble or practically insoluble salts, degree of solubility in water and occurrence of any precipitation process may be determi ...
... substances are soluble in water and other polar solvents while the non-polar, covalent compounds are more soluble in the non-polar solvents. In sparingly soluble, slightly soluble or practically insoluble salts, degree of solubility in water and occurrence of any precipitation process may be determi ...
Handbook of Inorganic Chemicals
... substances are soluble in water and other polar solvents while the non-polar, covalent compounds are more soluble in the non-polar solvents. In sparingly soluble, slightly soluble or practically insoluble salts, degree of solubility in water and occurrence of any precipitation process may be determi ...
... substances are soluble in water and other polar solvents while the non-polar, covalent compounds are more soluble in the non-polar solvents. In sparingly soluble, slightly soluble or practically insoluble salts, degree of solubility in water and occurrence of any precipitation process may be determi ...
Evaluated kinetic and photochemical data for atmospheric chemistry
... based on consideration of suitability of experimental method, coverage of parameter space (temperature, partial pressure of gas phase species, humidity, concentrations in the condensed phase), within the atmospherically relevant range of variability. The general approach and methods used have been r ...
... based on consideration of suitability of experimental method, coverage of parameter space (temperature, partial pressure of gas phase species, humidity, concentrations in the condensed phase), within the atmospherically relevant range of variability. The general approach and methods used have been r ...
Chapter 4 Solution Manual
... when the compound is dissolved in water. Covalent compounds with polar bonds are often soluble in water since the polar bonds of the covalent compound interact with those in water. Solution: a) Lithium nitrate is an ionic compound and is expected to be soluble in water. b) Glycine (H 2 NCH 2 COOH) i ...
... when the compound is dissolved in water. Covalent compounds with polar bonds are often soluble in water since the polar bonds of the covalent compound interact with those in water. Solution: a) Lithium nitrate is an ionic compound and is expected to be soluble in water. b) Glycine (H 2 NCH 2 COOH) i ...
Document
... • One can determine values of k and exponents m and n in several ways: 1. Keep the initial concentration of B constant while varying the initial concentration of A and calculating the initial rate of the reaction and then deducing the order of the reaction with respect to A 2. Determine the order of ...
... • One can determine values of k and exponents m and n in several ways: 1. Keep the initial concentration of B constant while varying the initial concentration of A and calculating the initial rate of the reaction and then deducing the order of the reaction with respect to A 2. Determine the order of ...
Chalogen-Nitrogen Chemistry
... conform to the well known Hückel (4n + 2)-electron rule of organic chemistry. This suggestion, which was based on simple electroncounting concepts, provided an additional impetus for both experimental and theoretical investigations of S–N systems. The classic book in this field “The Inorganic Heter ...
... conform to the well known Hückel (4n + 2)-electron rule of organic chemistry. This suggestion, which was based on simple electroncounting concepts, provided an additional impetus for both experimental and theoretical investigations of S–N systems. The classic book in this field “The Inorganic Heter ...
vysoké učení technické v brně
... In chemistry, carbene is a highly reactive organic molecule having a divalent carbon atom with only six valence electrons which describes the several formula of R1R2C: (two substituents and two electrons) 1 . By definition, divalent carbons utilize only two of the four bonds, which are capable of bo ...
... In chemistry, carbene is a highly reactive organic molecule having a divalent carbon atom with only six valence electrons which describes the several formula of R1R2C: (two substituents and two electrons) 1 . By definition, divalent carbons utilize only two of the four bonds, which are capable of bo ...
volume 2 - HotNews
... International Chemistry Olympiads (ICHO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty ICHOs. The whole review of the competition tasks set in the ICHO in its fourty-year histor ...
... International Chemistry Olympiads (ICHO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty ICHOs. The whole review of the competition tasks set in the ICHO in its fourty-year histor ...
volume 2 - PianetaChimica
... International Chemistry Olympiads (IChO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty IChOs. The whole review of the competition tasks set in the IChO in its fourty-year histor ...
... International Chemistry Olympiads (IChO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty IChOs. The whole review of the competition tasks set in the IChO in its fourty-year histor ...
Chapter 4 - Chemistry
... Strategy: In order to break a redox reaction down into an oxidation half-reaction and a reduction halfreaction, you should first assign oxidation numbers to all the atoms in the reaction. In this way, you can determine which element is oxidized (loses electrons) and which element is reduced (gains e ...
... Strategy: In order to break a redox reaction down into an oxidation half-reaction and a reduction halfreaction, you should first assign oxidation numbers to all the atoms in the reaction. In this way, you can determine which element is oxidized (loses electrons) and which element is reduced (gains e ...
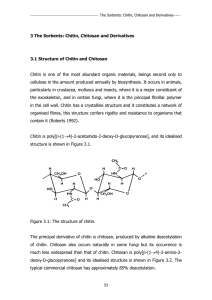
3 The Sorbents: Chitin, Chitosan and Derivatives
... interact with metal ions. It is also well known that chitosan can dissolve in either organic or mineral acids. This is a restrictive parameter for industrial applications in waste water treatment. A crosslinking procedure can be used to reinforce the chemical and mechanical properties of chitosan us ...
... interact with metal ions. It is also well known that chitosan can dissolve in either organic or mineral acids. This is a restrictive parameter for industrial applications in waste water treatment. A crosslinking procedure can be used to reinforce the chemical and mechanical properties of chitosan us ...
Chapter 4 - Chemistry
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
... Strategy: Hydrogen displacement: Any metal above hydrogen in the activity series will displace it from water or from an acid. Metals below hydrogen will not react with either water or an acid. Solution: Only (b) Li and (d) Ca are above hydrogen in the activity series, so they are the only metals in ...
COMPETITION PTOBLEMS 1
... them such a form that they may be used in practice and further chemical education. Consequently, it was necessary to make some corrections in order to unify the form of the problems. However, they did not concern the contents and language of the problems. Many of the first problems were published se ...
... them such a form that they may be used in practice and further chemical education. Consequently, it was necessary to make some corrections in order to unify the form of the problems. However, they did not concern the contents and language of the problems. Many of the first problems were published se ...
APPLIED PHYSICS REVIEWS Ion and electron irradiation
... immediately after the impact 共on the picosecond time scale兲, but some defects may remain in the system or form more complicated defect structures. The slowing down of an energetic ion moving in a solid target can be separated into two different mechanisms:83,84 electronic and nuclear stopping. The n ...
... immediately after the impact 共on the picosecond time scale兲, but some defects may remain in the system or form more complicated defect structures. The slowing down of an energetic ion moving in a solid target can be separated into two different mechanisms:83,84 electronic and nuclear stopping. The n ...