Reaction Rates/Chemical Kinetics
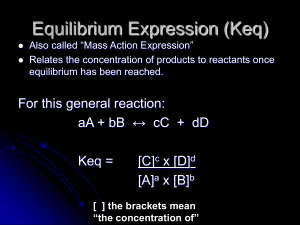
... Analyze: We are asked to write the equilibrium-constant expression for a reaction and to determine the value of Kc given the chemical equation and equilibrium constant for the reverse reaction. Plan: The equilibrium-constant expression is a quotient of products over reactants, each raised to a power ...
... Analyze: We are asked to write the equilibrium-constant expression for a reaction and to determine the value of Kc given the chemical equation and equilibrium constant for the reverse reaction. Plan: The equilibrium-constant expression is a quotient of products over reactants, each raised to a power ...
The coordination chemistry of pyridyl oximes
... This is the commonly used method for the synthesis of new oxime ligands by coordination chemists (Eq. 3). It has been shown that the rate of formation of oximes is maximum at a pH which depends on the substrate ...
... This is the commonly used method for the synthesis of new oxime ligands by coordination chemists (Eq. 3). It has been shown that the rate of formation of oximes is maximum at a pH which depends on the substrate ...
KINETICS (chap 12)
... Apply le Chatelier's principle – particularly it’s impact on K or the conc of a molecule after an add/loss of another molecule or a temperature or pressure change. Be able to use H (heat and temp) in le Chatelier's principle and K. Solve I.C.E. problems. Also know how to do ICE if your given amount ...
... Apply le Chatelier's principle – particularly it’s impact on K or the conc of a molecule after an add/loss of another molecule or a temperature or pressure change. Be able to use H (heat and temp) in le Chatelier's principle and K. Solve I.C.E. problems. Also know how to do ICE if your given amount ...
PDF File
... allow us to detect structural changes of this ribozyme at the level of individual molecular interactions+ There is evidence for a conformational change within the active site upon binding of the oligonucleotide substrate and the guanosine nucleophile+ The presence of bound S at the active site incre ...
... allow us to detect structural changes of this ribozyme at the level of individual molecular interactions+ There is evidence for a conformational change within the active site upon binding of the oligonucleotide substrate and the guanosine nucleophile+ The presence of bound S at the active site incre ...
Unit 6: Solution Chemistry Content Outline: Basic Solution Chemistry
... For example, cold tea is much harder to sweeten than hot tea. 3. Gases also require a specified pressure…remember that temperature directly affects pressure. For example, cold water holds more dissolved Oxygen gas than warm water. The amount of dissolved Oxygen gas determines the types of life forms ...
... For example, cold tea is much harder to sweeten than hot tea. 3. Gases also require a specified pressure…remember that temperature directly affects pressure. For example, cold water holds more dissolved Oxygen gas than warm water. The amount of dissolved Oxygen gas determines the types of life forms ...
Eötvös Loránd Science University Faculty of Sciences Department of
... kinetics. Potential energy surfaces in reactive systems. The transition state theory based on quasi-equilibrium approach. Alternative theories of bimolecular reactions. Calculating rate constants for elementary reactions, and the isotope effect. Formal kinetics of chemical reactions. Rate equations ...
... kinetics. Potential energy surfaces in reactive systems. The transition state theory based on quasi-equilibrium approach. Alternative theories of bimolecular reactions. Calculating rate constants for elementary reactions, and the isotope effect. Formal kinetics of chemical reactions. Rate equations ...
Substituent groups in aryl- and arylalkylphosphanes: effects on
... Phosphanes contain a lone electron pair at the phosphorus atom, which is used for the formation of a σ-bond with metals. π-Back-bonding from the d-orbitals of metals in low oxidation states is important in the case of electron-rich metals. The P–R σ*-orbitals are utilized for π-back-bonding, and emp ...
... Phosphanes contain a lone electron pair at the phosphorus atom, which is used for the formation of a σ-bond with metals. π-Back-bonding from the d-orbitals of metals in low oxidation states is important in the case of electron-rich metals. The P–R σ*-orbitals are utilized for π-back-bonding, and emp ...
218 - Chimica
... that often lead to supramolecular isomers,1c,4 can more easily produce these new classes of compounds. We are currently investigating the use of the flexible ligand 1,4-bis(imidazol1-ylmethyl)benzene (bix), together with different MSO4 salts, since this spacer has already proven a certain ability to ...
... that often lead to supramolecular isomers,1c,4 can more easily produce these new classes of compounds. We are currently investigating the use of the flexible ligand 1,4-bis(imidazol1-ylmethyl)benzene (bix), together with different MSO4 salts, since this spacer has already proven a certain ability to ...
Growth of Platinum Clusters in Solution and on Biopolymers: The
... principles molecular dynamics techniques [11]. Our goal is to propose a microscopic mechanism for the formation of platinum nanoparticles after reduction of Pt(II) complexes, both free in solution and supported by biopolymers. Platinum clusters are of special interest because of their unique electro ...
... principles molecular dynamics techniques [11]. Our goal is to propose a microscopic mechanism for the formation of platinum nanoparticles after reduction of Pt(II) complexes, both free in solution and supported by biopolymers. Platinum clusters are of special interest because of their unique electro ...
Lithium, Sodium and Potassium Magnesiate Chemistry: A Structural
... replacement of a hydrogen atom with a metal one) has been known since 1908 when Schorigin reported that a C-H bond of benzene could be cleaved by a mixture of sodium metal and diethylmercury, to yield phenylsodium.1,2 Monometallic compounds, particularly organolithium reagents have historically been ...
... replacement of a hydrogen atom with a metal one) has been known since 1908 when Schorigin reported that a C-H bond of benzene could be cleaved by a mixture of sodium metal and diethylmercury, to yield phenylsodium.1,2 Monometallic compounds, particularly organolithium reagents have historically been ...
Interaction of an extended series of N-substituted di(2
... an investigation aimed at extending the Cu(II) chemistry of both the parent dipic ligand 1 and its N-substituted derivatives 2–9. With respect to this, it is noted that we have recently reported the results of an investigation of the complexation behaviour of a related series of N-substituted deriva ...
... an investigation aimed at extending the Cu(II) chemistry of both the parent dipic ligand 1 and its N-substituted derivatives 2–9. With respect to this, it is noted that we have recently reported the results of an investigation of the complexation behaviour of a related series of N-substituted deriva ...
5.2 Calculations of Enthalpy Changes (SL/HL)
... In an endothermic reaction, the reactants have less enthalpy than the products. There is an increase in energy taken from the surroundings so that H is positive and the products are less stable than the reactants. ...
... In an endothermic reaction, the reactants have less enthalpy than the products. There is an increase in energy taken from the surroundings so that H is positive and the products are less stable than the reactants. ...
D:\MyFiles\general manual\techniques\recrystallization.wpd
... molecules of Y, and X will be added preferentially. Thus, crystals of pure X will form, and excess Y is left behind in solution. Recrystallizing from solvent mixture A-B Great in theory, but how does it work in practise? The easiest way of recrystallizing a compound is when the compound is very solu ...
... molecules of Y, and X will be added preferentially. Thus, crystals of pure X will form, and excess Y is left behind in solution. Recrystallizing from solvent mixture A-B Great in theory, but how does it work in practise? The easiest way of recrystallizing a compound is when the compound is very solu ...
Chapter 16
... is probably it contains anions: a. NO3– (nitrate), CH3CO2– (acetate, also written C2H3O2–) b. Cl–, Br–, I– (halides) except Ag+, Hg22+, Pb2+ halides c. SO42– (sulfate) except Ca2+, Sr2+, Ba2+, and Pb2+ sulfates Other ionic compounds are probably insoluble. ...
... is probably it contains anions: a. NO3– (nitrate), CH3CO2– (acetate, also written C2H3O2–) b. Cl–, Br–, I– (halides) except Ag+, Hg22+, Pb2+ halides c. SO42– (sulfate) except Ca2+, Sr2+, Ba2+, and Pb2+ sulfates Other ionic compounds are probably insoluble. ...
Higher Chemistry Learning Outcomes
... nuclei and the outer delocalised electrons in metal atoms. Atoms in a covalent bond are held together by electrostatic forces of attraction between positively charged nuclei and negatively charged shared electrons. The polarity of a covalent bond depends on the difference in electronegativity be ...
... nuclei and the outer delocalised electrons in metal atoms. Atoms in a covalent bond are held together by electrostatic forces of attraction between positively charged nuclei and negatively charged shared electrons. The polarity of a covalent bond depends on the difference in electronegativity be ...
Part II - American Chemical Society
... Determine the ratio of the pressure calculated in 3.b.ii. to this value. c. Gold can be extracted from its ores by reacting the ore with O2 gas in the presence of aqueous CN- ions according to this equation. 4Au(s) + 8CN-(aq) + O2(g) + 2H2O(l) s 4Au(CN)2-(aq) + 4OH-(aq) i. Write the equilibrium expr ...
... Determine the ratio of the pressure calculated in 3.b.ii. to this value. c. Gold can be extracted from its ores by reacting the ore with O2 gas in the presence of aqueous CN- ions according to this equation. 4Au(s) + 8CN-(aq) + O2(g) + 2H2O(l) s 4Au(CN)2-(aq) + 4OH-(aq) i. Write the equilibrium expr ...