Ch. 3: “Cell Structure”
... between the structure of a membrane protein and the lipid bilayer. The cause in this relationship is the nonpolar middle part of a membrane protein. What is the effect? ...
... between the structure of a membrane protein and the lipid bilayer. The cause in this relationship is the nonpolar middle part of a membrane protein. What is the effect? ...
a sample task
... amino acids in a polypeptide chain. For example, the pancreatic hormone insulin has two polypeptide chains, A and B, shown in the diagram below. Each chain has its own set of amino acids, assembled in a particular order. For instance, the sequence of the A chain starts with glycine at the N-terminus ...
... amino acids in a polypeptide chain. For example, the pancreatic hormone insulin has two polypeptide chains, A and B, shown in the diagram below. Each chain has its own set of amino acids, assembled in a particular order. For instance, the sequence of the A chain starts with glycine at the N-terminus ...
BIOCHEMISTRY - Mexico Central School District
... Other Functions: • key components of cell membranes • Steroids are special lipids used to build many reproductive hormones and cholesterol ...
... Other Functions: • key components of cell membranes • Steroids are special lipids used to build many reproductive hormones and cholesterol ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 21. Briefly write the working principle of centrifuge and chromatography. 22. Explain how carbohydrate molecules are classified? 23. Give brief account on different types of gel used in electrophoresis. 24. Write short notes on alkaloids and flavanoids. 25. Give an account of classification of enzym ...
... 21. Briefly write the working principle of centrifuge and chromatography. 22. Explain how carbohydrate molecules are classified? 23. Give brief account on different types of gel used in electrophoresis. 24. Write short notes on alkaloids and flavanoids. 25. Give an account of classification of enzym ...
Chemistry of Life
... Secondary: Beta or Alpha bonds Tertiary: Folded polypeptide chain Quaternary: Many polypeptide chains together ...
... Secondary: Beta or Alpha bonds Tertiary: Folded polypeptide chain Quaternary: Many polypeptide chains together ...
No Slide Title
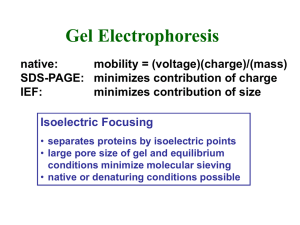
... • polyester screens separate chamber into 20 compartments • fractions rapidly harvested following electrofocusing ...
... • polyester screens separate chamber into 20 compartments • fractions rapidly harvested following electrofocusing ...
ORGANIC COMPOUNDS
... Order of bases determine what amino acids sequence is used in protein function of individual proteins ...
... Order of bases determine what amino acids sequence is used in protein function of individual proteins ...
Protein Synthesis (Translation)
... Translation or protein synthesis is the process of making a protein Proteins are made up of amino acids (small building blocks) There are 20 different types of amino acids ...
... Translation or protein synthesis is the process of making a protein Proteins are made up of amino acids (small building blocks) There are 20 different types of amino acids ...
Chapter 3 (part 2) – Protein Function
... • Enzymes and bound ligand go through a number of intermediate forms of different geometry. They are all called transition states. • The energy that it takes to get to the most unstable transition state is called the activation energy. • Enzymes speed reactions by selectively stabilizing the transi ...
... • Enzymes and bound ligand go through a number of intermediate forms of different geometry. They are all called transition states. • The energy that it takes to get to the most unstable transition state is called the activation energy. • Enzymes speed reactions by selectively stabilizing the transi ...
Lecture 7 Proteins 1. Which amino acids are considered as acidic
... sulphate is the most commonly used salt as it is cheap and sufficiently soluble. Other salts which can be used are ammonium acetate, sodium sulphate, and sodium citrate. 6. How to differentiate between secondary and tertiary structure of proteins? Answer: Tertiary protein structure refers to the com ...
... sulphate is the most commonly used salt as it is cheap and sufficiently soluble. Other salts which can be used are ammonium acetate, sodium sulphate, and sodium citrate. 6. How to differentiate between secondary and tertiary structure of proteins? Answer: Tertiary protein structure refers to the com ...
Cell Bio/Physio Lecture 6 Objectives Sunday, August 14, 2011 11:41
... opportunity for cooperative binding of ligands (i.e. O2 binding to hemoglobin), form binding sites for complex molecules (i.e. antigen binding to immunoglobin), and increase stability of the protein. The polypeptide chains of fibrous proteins such as collagen are aligned along an axis, have repeatin ...
... opportunity for cooperative binding of ligands (i.e. O2 binding to hemoglobin), form binding sites for complex molecules (i.e. antigen binding to immunoglobin), and increase stability of the protein. The polypeptide chains of fibrous proteins such as collagen are aligned along an axis, have repeatin ...
Key to Exam 2
... conjunction with gel electrophoresis to identify specific proteins or subunits recognized by antibodies. Many times the two methods will provide the same information about a protein and can be used interchangeably. In other situations one of the methods will work better or be more appropriate. For e ...
... conjunction with gel electrophoresis to identify specific proteins or subunits recognized by antibodies. Many times the two methods will provide the same information about a protein and can be used interchangeably. In other situations one of the methods will work better or be more appropriate. For e ...
Biochemistry Notes 2012
... • Elements – pure substances that can’t be broken down into other substances. (atoms) • Molecules – two or more atoms joined together by chemical bonds. (smallest combination that can’t be divided without changing its chemical and physical properties) • Compounds –substances composed of atoms of dif ...
... • Elements – pure substances that can’t be broken down into other substances. (atoms) • Molecules – two or more atoms joined together by chemical bonds. (smallest combination that can’t be divided without changing its chemical and physical properties) • Compounds –substances composed of atoms of dif ...
Chemistry of Life Carbohydrates Lipids Nucleic Acids ATP – The
... and carboxylic acid (COOH),along with a side-chain specific to each amino acid.] The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen. Proteins are macromolecules made from amino acids (the subunit) binding together. About 500 amino acids are known. ...
... and carboxylic acid (COOH),along with a side-chain specific to each amino acid.] The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen. Proteins are macromolecules made from amino acids (the subunit) binding together. About 500 amino acids are known. ...
Towards a More Effective Anticancer Therapy By Mariam Ludim
... What types of chemical interactions exist between two proteins? What changes occur in proteins when they interact with each other? One way to prevent cancer from multiplying is to avoid cancer cells from dividing. Our goal is to achieve the interruption or inhibition of interactions between proteins ...
... What types of chemical interactions exist between two proteins? What changes occur in proteins when they interact with each other? One way to prevent cancer from multiplying is to avoid cancer cells from dividing. Our goal is to achieve the interruption or inhibition of interactions between proteins ...
Chapter 14 Oxidative Phosphorylation Prokaryotes are bacteria
... Eukaryotes contain multiple chromosomes surrounded by a membrane (nucleus) and membrane-bound organelles. Some organelles such as the nucleus and mitochondrion have two membranes. Animal Cell ...
... Eukaryotes contain multiple chromosomes surrounded by a membrane (nucleus) and membrane-bound organelles. Some organelles such as the nucleus and mitochondrion have two membranes. Animal Cell ...
CHAPTER 5 THE STRUCTURE AND FUNCTION OF LARGE
... 17. Name the two ends of a protein and explain the reason for their names. 18. List and describe the four major components of an amino acid. Explain how amino acids may be grouped according to the physical and chemical properties of the R group. 19. Explain what determines protein structure and why ...
... 17. Name the two ends of a protein and explain the reason for their names. 18. List and describe the four major components of an amino acid. Explain how amino acids may be grouped according to the physical and chemical properties of the R group. 19. Explain what determines protein structure and why ...
macromolecules
... • Carbon compounds that come from living organisms are called organic compounds. • Two carbon atoms can form various types of covalent bonds—single, double or triple. ...
... • Carbon compounds that come from living organisms are called organic compounds. • Two carbon atoms can form various types of covalent bonds—single, double or triple. ...
Spec for students digestion and metabolism
... Year 4 Biology – Digestion and Metabolism! 4.2.2.1 The human digestive system This section assumes knowledge of the digestive system studied in Key Stage 3 science. The digestive system is an example of an organ system in which several organs work together to digest and absorb food. Students should ...
... Year 4 Biology – Digestion and Metabolism! 4.2.2.1 The human digestive system This section assumes knowledge of the digestive system studied in Key Stage 3 science. The digestive system is an example of an organ system in which several organs work together to digest and absorb food. Students should ...
Ch6PROTEIN
... • Albumin transports a variety of nutrients such as calcium, zinc, and Vitamin B6 • Transferrin transports iron (hemoglobin – a protein, contains iron, but it transports oxygen) • Proteins may also acts as channels or pumps across the cell membrane Energy Source • If the diet does not provide enough ...
... • Albumin transports a variety of nutrients such as calcium, zinc, and Vitamin B6 • Transferrin transports iron (hemoglobin – a protein, contains iron, but it transports oxygen) • Proteins may also acts as channels or pumps across the cell membrane Energy Source • If the diet does not provide enough ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.



![Proteins2[1]](http://s1.studyres.com/store/data/008291804_1-fc4b593e0423ea377f021b9f7071accd-300x300.png)