Biochemistry - Bishop Ireton High School
... • Amino group- NH2 • Carboxyl group-COOH • R group- R groups are different for each of the 20 amino acids. ...
... • Amino group- NH2 • Carboxyl group-COOH • R group- R groups are different for each of the 20 amino acids. ...
Protein - manorhousehomeeconomics
... Proteins are made up of atoms of: Carbon C Hydrogen H Oxygen O Nitrogen N and sometimes small amounts of Phosphorus (P), Sulphur (S) and Iron (Fe) Nitrogen is needed for growth. Proteins are the only nutrients that contain the element nitrogen. These elements are bonded together in s ...
... Proteins are made up of atoms of: Carbon C Hydrogen H Oxygen O Nitrogen N and sometimes small amounts of Phosphorus (P), Sulphur (S) and Iron (Fe) Nitrogen is needed for growth. Proteins are the only nutrients that contain the element nitrogen. These elements are bonded together in s ...
The Subcellular Distribution of Multigene Family 110 Proteins of
... their internal envelopes through enwrapment by membranes derived from the endoplasmic reticulum (ER). However, the mechanisms that underlie the formation of viral factories and progenitor viral membranes are as yet unclear. Analysis of the published genome of the virus revealed a conserved multigene ...
... their internal envelopes through enwrapment by membranes derived from the endoplasmic reticulum (ER). However, the mechanisms that underlie the formation of viral factories and progenitor viral membranes are as yet unclear. Analysis of the published genome of the virus revealed a conserved multigene ...
Secondary structure
... Peptidyl polymers • A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. • Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
... Peptidyl polymers • A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. • Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
BEST - Doral Academy Preparatory
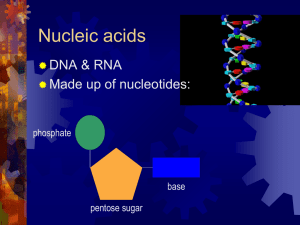
... of C, O, H, N and P. Contain instructions cells need for life. a.) DNA (Deoxyribonucleic acid): genetic material carried from parent to offspring. b.) RNA (Ribonucleic acid): plays a role in the production of proteins. ...
... of C, O, H, N and P. Contain instructions cells need for life. a.) DNA (Deoxyribonucleic acid): genetic material carried from parent to offspring. b.) RNA (Ribonucleic acid): plays a role in the production of proteins. ...
DAAM1 antibody - middle region (ARP55131_P050)
... positive control. Aviva Systems Biology strives to provide antibodies covering each member of a whole protein family of your interest. We also use our best efforts to provide you antibodies recognize various epitopes of a target protein. For availability of antibody needed for your experiment, pleas ...
... positive control. Aviva Systems Biology strives to provide antibodies covering each member of a whole protein family of your interest. We also use our best efforts to provide you antibodies recognize various epitopes of a target protein. For availability of antibody needed for your experiment, pleas ...
Heat shock protein (Hsp)65-70: dominant self
... the increased expression after exposure to elevated temperatures (assumed to provide the cells with protection during recovery from stress), but that now are identified as structurally conserved elements that are constitutively expressed in prokaryotic and eukaryotic organisms even in the absence of ...
... the increased expression after exposure to elevated temperatures (assumed to provide the cells with protection during recovery from stress), but that now are identified as structurally conserved elements that are constitutively expressed in prokaryotic and eukaryotic organisms even in the absence of ...
Protein PowerPoint - Bowdle FACS
... • Basic units are amino acids – “building blocks of protein” ...
... • Basic units are amino acids – “building blocks of protein” ...
Identification of HLA-A*0201-Restricted CD8+ Cytotoxic T
... pathogenic immune responses and/or fail to induce strong protective CD8+ T cell responses. T cell-mediated immune responses are believed to play an important role in controlling herpes infection; however, immunedominant responses from HSV envelope glycoproteins have not been enough to be proved as a ...
... pathogenic immune responses and/or fail to induce strong protective CD8+ T cell responses. T cell-mediated immune responses are believed to play an important role in controlling herpes infection; however, immunedominant responses from HSV envelope glycoproteins have not been enough to be proved as a ...
Powerpoint version
... Extracellular matrix - Biological “glue.” 3 protein fibers are interwoven in this matrix: collagen, elastin, fibronectin. Secreted by cells Desmosomes – “Rivets” to anchor adjacent cells that are not touching ...
... Extracellular matrix - Biological “glue.” 3 protein fibers are interwoven in this matrix: collagen, elastin, fibronectin. Secreted by cells Desmosomes – “Rivets” to anchor adjacent cells that are not touching ...
doc
... A. Visualize where a nucleotide cofactor binds. B. Compare the structures of two sequences. C. Color a structure by how closely it matches another. D. Detect homology with 100% certainty. E. Visualize the conformational change that the ATPsynthase undergoes during its catalytic cycle. 11. Which elem ...
... A. Visualize where a nucleotide cofactor binds. B. Compare the structures of two sequences. C. Color a structure by how closely it matches another. D. Detect homology with 100% certainty. E. Visualize the conformational change that the ATPsynthase undergoes during its catalytic cycle. 11. Which elem ...
Proteins
... chains. Each of these polypeptide chains have a primary, secondary and tertiary structure. Collagen (gives your skin its strength) is formed by several chain making like a rope. Hemoglobin (transports oxygen) is another example of quaternary structure protein. ...
... chains. Each of these polypeptide chains have a primary, secondary and tertiary structure. Collagen (gives your skin its strength) is formed by several chain making like a rope. Hemoglobin (transports oxygen) is another example of quaternary structure protein. ...
No Slide Title
... (they are both lipocalins, sharing the GXW motif). But they are distant relatives, and do not share significant amino acid identity in a pairwise alignment. ...
... (they are both lipocalins, sharing the GXW motif). But they are distant relatives, and do not share significant amino acid identity in a pairwise alignment. ...
Macromolecules Worksheet
... ____________________ 5. These are the individual subunits that make up DNA and RNA. ____________________ 6. What is a long chain of amino acids called? ____________________ 7. What sugar does DNA contain? ____________________ 8. When the pH is greater than 7, it is called this. ____________________ ...
... ____________________ 5. These are the individual subunits that make up DNA and RNA. ____________________ 6. What is a long chain of amino acids called? ____________________ 7. What sugar does DNA contain? ____________________ 8. When the pH is greater than 7, it is called this. ____________________ ...
The Genetic Code
... amino acid this codon codes for! – Each code always starts with AUG (start) and ends with a stop codon! ...
... amino acid this codon codes for! – Each code always starts with AUG (start) and ends with a stop codon! ...
notes File - selu moodle
... Secondary – folding due to Hydrogen bonds attracting amino acids (can cause standard shapes of alpha helix, or beta sheet) Tertiary – folding due to hydrophobic exclusion, ionic bonds and disulfide bonds (bonds between functional groups with S). Quaternary – bond to another structure Shape is more i ...
... Secondary – folding due to Hydrogen bonds attracting amino acids (can cause standard shapes of alpha helix, or beta sheet) Tertiary – folding due to hydrophobic exclusion, ionic bonds and disulfide bonds (bonds between functional groups with S). Quaternary – bond to another structure Shape is more i ...
Click here to go back
... cell division the DNA must replicate The enzyme helicase unwinds the DNA double helix The exposed bases bind to free floating nucleotides in the nucleoplasm DNA polymerase binds the complimentary nucleotides Replication is semiconservative ...
... cell division the DNA must replicate The enzyme helicase unwinds the DNA double helix The exposed bases bind to free floating nucleotides in the nucleoplasm DNA polymerase binds the complimentary nucleotides Replication is semiconservative ...
Chapter Twenty-Seven: Amino Acids
... o Rinse solid support o Add second N-protected amino acid via C-terminus ...
... o Rinse solid support o Add second N-protected amino acid via C-terminus ...
Biomolecules I. Introduction. - biochemistry: study of chemical
... contributing to a specific tertiary structure; some also display quaternary structure. - usually water soluble, mobile, chemically active; crucial in all biological processes, most are functional proteins. D. Enzymes and enzyme activity. 1. General comments: - enzymes are globular proteins, act as b ...
... contributing to a specific tertiary structure; some also display quaternary structure. - usually water soluble, mobile, chemically active; crucial in all biological processes, most are functional proteins. D. Enzymes and enzyme activity. 1. General comments: - enzymes are globular proteins, act as b ...
An Introduction to Protein Structure Databases
... and orientation (no connectivities between the secondary structures) Topology (FOLD family): overall shape and connectivities. Homologous superfamily: proteins are thought to share common ancestor. Similarities by sequence alignment and then by structure comparison using the SSAP structural align ...
... and orientation (no connectivities between the secondary structures) Topology (FOLD family): overall shape and connectivities. Homologous superfamily: proteins are thought to share common ancestor. Similarities by sequence alignment and then by structure comparison using the SSAP structural align ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.