Gene Section S100B (S100 calcium binding protein B) in Oncology and Haematology
... effect seems to be dependent on the concentration of S100B and occurs at nanomolar concentrations. But micromolar levels of extracellular S100B stimulate apoptosis in vitro. Calcium binding induces a conformational change in S100B that allows the interaction with a variety of target proteins. These ...
... effect seems to be dependent on the concentration of S100B and occurs at nanomolar concentrations. But micromolar levels of extracellular S100B stimulate apoptosis in vitro. Calcium binding induces a conformational change in S100B that allows the interaction with a variety of target proteins. These ...
Problem 2
... code for the protein I picked, human serum transferring, 1d3k. PDB Sum gives a complete analysis of the protein structure, including domains and motifs. I found the protein to consist of two domains: ...
... code for the protein I picked, human serum transferring, 1d3k. PDB Sum gives a complete analysis of the protein structure, including domains and motifs. I found the protein to consist of two domains: ...
SQUADS #4
... that allows them to have their lowest-energy shapes. Which of the following statements about the proteins is most consistent with the information presented in the passage? A. If Scientist 1 is correct, all of the proteins will have their active shapes. B. If Scientist 1 is correct, all of the protei ...
... that allows them to have their lowest-energy shapes. Which of the following statements about the proteins is most consistent with the information presented in the passage? A. If Scientist 1 is correct, all of the proteins will have their active shapes. B. If Scientist 1 is correct, all of the protei ...
Protein structure prediction Haixu Tang School of Informatics
... are based on neural networks. The overall idea is that neural networks can be trained to recognize amino acid patterns in known secondary structure units, and to use these patterns to distinguish between the different types of secondary structure. Neural networks classify “input vectors” or “example ...
... are based on neural networks. The overall idea is that neural networks can be trained to recognize amino acid patterns in known secondary structure units, and to use these patterns to distinguish between the different types of secondary structure. Neural networks classify “input vectors” or “example ...
Bottom-up Nanobiotechnology
... The mammalian nose has the ability to rapidly distinguish between an enormous range of small molecules at low concentrations ...
... The mammalian nose has the ability to rapidly distinguish between an enormous range of small molecules at low concentrations ...
TD7: Gel Electrophoresis Photoaffinity probes GEL
... - autoradiography or phosphorimaging (if the protein is radiolabeled) - Coomassie stain (most common, cheap) binds to Arg, Trp, Tyr, His, Phe gives blue bands - Sliver Stain (more sensitive than Coomassie) proteins reduce Arg-1 to Ag metal - Immunoblot (transfer proteins from gel to a membrane, dete ...
... - autoradiography or phosphorimaging (if the protein is radiolabeled) - Coomassie stain (most common, cheap) binds to Arg, Trp, Tyr, His, Phe gives blue bands - Sliver Stain (more sensitive than Coomassie) proteins reduce Arg-1 to Ag metal - Immunoblot (transfer proteins from gel to a membrane, dete ...
Mr. David Cortens In Vivo Synthesis of ?Click? Functionalized
... with the natural transcriptional/ translational machinery of S. cerevisiae (2,7), it can be added to the genetic repertoire of the yeast and therefore encode for a 21st (non-natural) amino acid. 2.2 The Use of S. Cerevisiae Yeast combines the ease of microbial growth and the simplicity of manipulati ...
... with the natural transcriptional/ translational machinery of S. cerevisiae (2,7), it can be added to the genetic repertoire of the yeast and therefore encode for a 21st (non-natural) amino acid. 2.2 The Use of S. Cerevisiae Yeast combines the ease of microbial growth and the simplicity of manipulati ...
Ch. 2 – Bio Chem
... They work in cells to speed up chemical reactions. The enzyme attaches to a specific substrate and react to produce products. Examine the graphic to the right which shows the digestion of proteins in the intestine. Specific enzymes work to break the peptide bonds between amino acids and then free ...
... They work in cells to speed up chemical reactions. The enzyme attaches to a specific substrate and react to produce products. Examine the graphic to the right which shows the digestion of proteins in the intestine. Specific enzymes work to break the peptide bonds between amino acids and then free ...
I - Decatur ISD
... Proteins are building blocks of structures called _______________________. Proteins are what your DNA codes to make A peptide bond forms between amino acids by dehydration synthesis. ____________________________= the building up of large molecules by removing water molecules Enzymes A. Speci ...
... Proteins are building blocks of structures called _______________________. Proteins are what your DNA codes to make A peptide bond forms between amino acids by dehydration synthesis. ____________________________= the building up of large molecules by removing water molecules Enzymes A. Speci ...
File
... • Proteins begin to fold after the amino acid chain winds away from the ribosome – First few amino acids in a protein secreted in a membrane form a “signal sequence” • Leads it and the ribosome into a pore in the ER membrane • Not found on proteins synthesized on free ribosomes ...
... • Proteins begin to fold after the amino acid chain winds away from the ribosome – First few amino acids in a protein secreted in a membrane form a “signal sequence” • Leads it and the ribosome into a pore in the ER membrane • Not found on proteins synthesized on free ribosomes ...
atom
... – Elements differ in the number of subatomic particles in their atoms. • The number of protons, the atomic number, determines which ...
... – Elements differ in the number of subatomic particles in their atoms. • The number of protons, the atomic number, determines which ...
Hypothesis-Driven Science Hypothesis
... – Elements differ in the number of subatomic particles in their atoms. • The number of protons, the atomic number, determines which ...
... – Elements differ in the number of subatomic particles in their atoms. • The number of protons, the atomic number, determines which ...
2.Molecular basis of heredity. Realization of hereditary information
... Proteins are prime examples of the buildingblock type of molecule. The monomers in this case are called amino acids. String an arbitrary number of them together in a chain—and you have a polypeptide; when the polypeptide folds up in a specific three-dimensional manner, you have a protein. As a pract ...
... Proteins are prime examples of the buildingblock type of molecule. The monomers in this case are called amino acids. String an arbitrary number of them together in a chain—and you have a polypeptide; when the polypeptide folds up in a specific three-dimensional manner, you have a protein. As a pract ...
Protein Synthesis Bead Activity
... __________________________________ and it occurs in the ______________________ of cells. mRNA leaves the nucleus to find a _______________. Next, we start the second part of protein synthesis called _____________________________ and it happens in the _____________________ of cells. During this proce ...
... __________________________________ and it occurs in the ______________________ of cells. mRNA leaves the nucleus to find a _______________. Next, we start the second part of protein synthesis called _____________________________ and it happens in the _____________________ of cells. During this proce ...
Biochemistry Review
... 19. Can proteins act as an energy source if there is shortage of carbohydrates and lipids? Yes 20. Describe the five functions of proteins? Structural: support as connective tissue and keratin for hair and nails; Transport: hemoglobin transports Oxygen in blood; Hormone: coordinate body activities, ...
... 19. Can proteins act as an energy source if there is shortage of carbohydrates and lipids? Yes 20. Describe the five functions of proteins? Structural: support as connective tissue and keratin for hair and nails; Transport: hemoglobin transports Oxygen in blood; Hormone: coordinate body activities, ...
2,3-BPG and the O 2
... O2-bound Fe acts rather as a Fe3+- O2- (superoxide anion) complex (chargetransfer complex, mixture of resonance structures); it is crucial to release oxygen as O2 because superoxide is a reactive oxygen species (ROS) and can generate further harmful species that damage various cellular components ( ...
... O2-bound Fe acts rather as a Fe3+- O2- (superoxide anion) complex (chargetransfer complex, mixture of resonance structures); it is crucial to release oxygen as O2 because superoxide is a reactive oxygen species (ROS) and can generate further harmful species that damage various cellular components ( ...
Macromolecules
... • More specifically- polypeptides dipeptide Amino acids linked by peptide bonds ...
... • More specifically- polypeptides dipeptide Amino acids linked by peptide bonds ...
Macromolecules - Ms Kim`s Biology Class
... • AA made of C+H, Carboxyl group, Amino group + R group • 20 AA’s made from the different combo’s of the R group ...
... • AA made of C+H, Carboxyl group, Amino group + R group • 20 AA’s made from the different combo’s of the R group ...
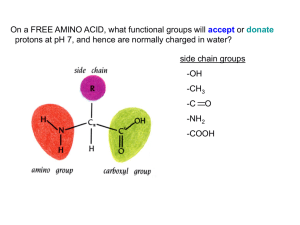
Lab 1 activity, AMINO ACIDS - Cal State LA
... Which amino acid side chains are positively charged at pH 7? ...
... Which amino acid side chains are positively charged at pH 7? ...
Three functionally diverged major structural proteins of white spot
... Nadala et al., 1998). The characterization of the structural proteins and their genomic sequence is of major importance to determine the taxonomic position of the virus. Furthermore, the structure and interaction of the WSSV virion proteins may explain the unique morphological features of this virus ...
... Nadala et al., 1998). The characterization of the structural proteins and their genomic sequence is of major importance to determine the taxonomic position of the virus. Furthermore, the structure and interaction of the WSSV virion proteins may explain the unique morphological features of this virus ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.