Slide 1 - MacWilliams Biology
... 1. First step in decoding genetic messages transcribe a nucleotide base sequence from DNA to RNA. 2. Transcribed information contains a code for making proteins. 3. Proteins are made by joining amino acids together into long chains, called polypeptides. 4. As many as 20 different amino acids are c ...
... 1. First step in decoding genetic messages transcribe a nucleotide base sequence from DNA to RNA. 2. Transcribed information contains a code for making proteins. 3. Proteins are made by joining amino acids together into long chains, called polypeptides. 4. As many as 20 different amino acids are c ...
Richards, F.M. The Protein Folding Problem. Scientific American, pp
... acids tend to be hydrophilic; they attract water molcculcs, which are quite polar. In contrast, nonpolar amino acids, which generally include hydrocarbon side chains, tend to be hydrophobic:they mix poorly with water and "prefer" to associatewith one other. Alternatively,one can think of them as bei ...
... acids tend to be hydrophilic; they attract water molcculcs, which are quite polar. In contrast, nonpolar amino acids, which generally include hydrocarbon side chains, tend to be hydrophobic:they mix poorly with water and "prefer" to associatewith one other. Alternatively,one can think of them as bei ...
Protein Concentration
... Pace, Vajdos, Lee, Grimsley and Gray, Protein Science, 4: 2411-2423, 1995 These extinction coefficients represent average values from a collection of folded proteins. The extinction coefficients for amino acid chromophores are sensitive to their environment, though the variation is relatively small ...
... Pace, Vajdos, Lee, Grimsley and Gray, Protein Science, 4: 2411-2423, 1995 These extinction coefficients represent average values from a collection of folded proteins. The extinction coefficients for amino acid chromophores are sensitive to their environment, though the variation is relatively small ...
General method for synthesis of azo dyes
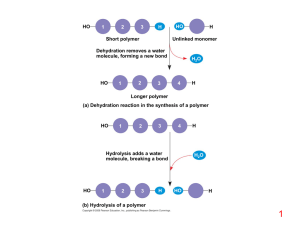
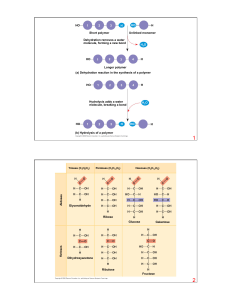
... • The protein is refluxed for about 24 hours. • This hydrolysis is the exact reverse of the formation of the peptide bond. • A molecule of water is in effect added across the linkage to regenerate the original amino acid and carboxyl groups. ...
... • The protein is refluxed for about 24 hours. • This hydrolysis is the exact reverse of the formation of the peptide bond. • A molecule of water is in effect added across the linkage to regenerate the original amino acid and carboxyl groups. ...
biochemistry - Bioscience High School
... Proteins are denatured when their 3-D shape changes. An incorrect shape can not bond with other molecules correctly and the enzyme does not function. Denaturalization occurs by ...
... Proteins are denatured when their 3-D shape changes. An incorrect shape can not bond with other molecules correctly and the enzyme does not function. Denaturalization occurs by ...
Chemistry 100 Quiz 6-
... For the protein's secondary structure, hydrogen bonds would shape the amino acid chain into either an alpha helix ( α-helix) or a beta pleated sheet ( β-pleated sheet). Sometimes both types of secondary +1 structure can exist on the same amino acid chain. ...
... For the protein's secondary structure, hydrogen bonds would shape the amino acid chain into either an alpha helix ( α-helix) or a beta pleated sheet ( β-pleated sheet). Sometimes both types of secondary +1 structure can exist on the same amino acid chain. ...
Biochemistry PowerPoint 1
... • Contain C, H, O, plus nitrogen • Formed from amino acids joined together • More than 20 amino acids can be joined in any order or number to make countless proteins (think of how many words can be made from 26 letters!) ...
... • Contain C, H, O, plus nitrogen • Formed from amino acids joined together • More than 20 amino acids can be joined in any order or number to make countless proteins (think of how many words can be made from 26 letters!) ...
GENE to PROTEIN
... Beadle and Tatum began to search for mutants of bread mold. They discovered that mutants differ from wild type in their nutritional needs. • Nutritional mutants are called auxotrophs. • Beadle and Tatum were able to demonstrate the relationship between genes and enzymes by studying mutant forms of b ...
... Beadle and Tatum began to search for mutants of bread mold. They discovered that mutants differ from wild type in their nutritional needs. • Nutritional mutants are called auxotrophs. • Beadle and Tatum were able to demonstrate the relationship between genes and enzymes by studying mutant forms of b ...
Ch.05The Structure and Function of Large Biological Molecules
... Normal red blood cells are full of individual hemoglobin moledules, each carrying oxygen. ...
... Normal red blood cells are full of individual hemoglobin moledules, each carrying oxygen. ...
Protein Synthesis
... TRANSCRIPTION IS OVER! All of this was happening in the nucleus. Now the final mRNA transcript leaves the nucleus and arrives at a ribosome in the cytoplasm for translation. ...
... TRANSCRIPTION IS OVER! All of this was happening in the nucleus. Now the final mRNA transcript leaves the nucleus and arrives at a ribosome in the cytoplasm for translation. ...
Ch.05The Structure and Function of Large Biological Molecules
... crystallize into a fiber; capacity to carry oxygen is greatly reduced. ...
... crystallize into a fiber; capacity to carry oxygen is greatly reduced. ...
Ch.05The Structure and Function of Large Biological Molecules
... cells are full of individual hemoglobin moledules, each carrying oxygen. ...
... cells are full of individual hemoglobin moledules, each carrying oxygen. ...
Plasmodesmata 2004. Surfing the Symplasm
... pathogen attack. Plasmodesmata (PDs; singular plasmodesma), plasma membrane-lined channels that cross the cell wall, are key components of this intercellular communication network. Historically, PDs were largely viewed as little more than channels that allowed the passive movement of small molecules ...
... pathogen attack. Plasmodesmata (PDs; singular plasmodesma), plasma membrane-lined channels that cross the cell wall, are key components of this intercellular communication network. Historically, PDs were largely viewed as little more than channels that allowed the passive movement of small molecules ...
Part I- Protein Purification
... Vo: void volume Ve: the volume of solvent required to elute solute (protein etc) from the column. Ve/Vo: relative elution volume, which is independent of the size of the particular column used. ...
... Vo: void volume Ve: the volume of solvent required to elute solute (protein etc) from the column. Ve/Vo: relative elution volume, which is independent of the size of the particular column used. ...
E-site
... • A- site: where amino-acylated tRNAs are deposited by EF-Tu when appropriate codon-anticodon pairing occurs • P-site: where the tRNA is bound to a polypeptide as opposed to a single amino acid • E-site: where a spent tRNA goes before getting kicked out ...
... • A- site: where amino-acylated tRNAs are deposited by EF-Tu when appropriate codon-anticodon pairing occurs • P-site: where the tRNA is bound to a polypeptide as opposed to a single amino acid • E-site: where a spent tRNA goes before getting kicked out ...
n-formyl methionine
... Formylmethionine (fMet) is an amino acid found in all living cells. It is a derivative of the amino acid methionine. It is a modified form of methionine in which a formyl group has been added to methionine's amino group. It plays a crucial part in the protein synthesis of bacteria, mitochondria and ...
... Formylmethionine (fMet) is an amino acid found in all living cells. It is a derivative of the amino acid methionine. It is a modified form of methionine in which a formyl group has been added to methionine's amino group. It plays a crucial part in the protein synthesis of bacteria, mitochondria and ...
the chemical constituents of cells constituents include
... Amino Acids • man can only synthesize about 10 kinds of amino acids • non-essential amino acids are those amino acids that can be synthesized by the body • essential amino acids are those amino acids that cannot be synthesized by the body and ...
... Amino Acids • man can only synthesize about 10 kinds of amino acids • non-essential amino acids are those amino acids that can be synthesized by the body • essential amino acids are those amino acids that cannot be synthesized by the body and ...
No Slide Title
... • Group of residues with high contact density, number of contacts within domains is higher than the number of contacts between domains. • A stable unit of protein structure that can fold autonomously • A rigid body linked to other domains by flexible linkers • A portion of the protein that can be ac ...
... • Group of residues with high contact density, number of contacts within domains is higher than the number of contacts between domains. • A stable unit of protein structure that can fold autonomously • A rigid body linked to other domains by flexible linkers • A portion of the protein that can be ac ...
Pfam-A
... • Group of residues with high contact density, number of contacts within domains is higher than the number of contacts between domains. • A stable unit of protein structure that can fold autonomously • A rigid body linked to other domains by flexible linkers • A portion of the protein that can be ac ...
... • Group of residues with high contact density, number of contacts within domains is higher than the number of contacts between domains. • A stable unit of protein structure that can fold autonomously • A rigid body linked to other domains by flexible linkers • A portion of the protein that can be ac ...
for first midterm
... Describe the structure of microtubules and at least two situations in which they play an important role. Describe the structure of microfilaments and at least two situations in which cells use them. Contrast the effects of inhibiting microtubule function with those caused by inhibiting microfilamen ...
... Describe the structure of microtubules and at least two situations in which they play an important role. Describe the structure of microfilaments and at least two situations in which cells use them. Contrast the effects of inhibiting microtubule function with those caused by inhibiting microfilamen ...
Table S1. Entities that were analyzed in the pathways shown in
... Table S1. Entities that were analyzed in the pathways shown in Figure 4 Name CD24 ...
... Table S1. Entities that were analyzed in the pathways shown in Figure 4 Name CD24 ...
Lecture_3. ppt - Department of Molecular & Cell Biology
... Western blotting (Immunoblotting) - Identification of protein antigen following SDS-PAGE ...
... Western blotting (Immunoblotting) - Identification of protein antigen following SDS-PAGE ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.