comprehensive biochemistry
... citrate synthase, 24 - (Hi) Different induction patterns for the same overall metabolic pathway, 25 — f. CO 2 fixation in chemo- and photo-autotrophic bacteria (i) Mechanism of CO 2 incorporation, 26 - (i/) ATP production in photoautotrophs; photosystems I and II, 27 g. Biosynthesis of tetrapyrroles ...
... citrate synthase, 24 - (Hi) Different induction patterns for the same overall metabolic pathway, 25 — f. CO 2 fixation in chemo- and photo-autotrophic bacteria (i) Mechanism of CO 2 incorporation, 26 - (i/) ATP production in photoautotrophs; photosystems I and II, 27 g. Biosynthesis of tetrapyrroles ...
Oksenberg_N_bms265ppt
... How: Purifying rat liver coated vesicles, ovoid structures that were smaller than most coated vesicles were observed. ...
... How: Purifying rat liver coated vesicles, ovoid structures that were smaller than most coated vesicles were observed. ...
ISOLATION OF A BASIC LECTIN FROM SARGASSUM
... in 0.15 M NaCl and treated with ammonium sulphate (20% of saturation). HA of 020% fraction (F0-20%) was evaluated at different temperatures (30-100 ºC) and pH values (6.0 -10.0), as well as at presence of monosaccharides and cations (20 mM MgCl2 and CaCl2). To isolate the lectin Sephadex G-100 chrom ...
... in 0.15 M NaCl and treated with ammonium sulphate (20% of saturation). HA of 020% fraction (F0-20%) was evaluated at different temperatures (30-100 ºC) and pH values (6.0 -10.0), as well as at presence of monosaccharides and cations (20 mM MgCl2 and CaCl2). To isolate the lectin Sephadex G-100 chrom ...
Chapter 5 Problem set
... A. Oligosaccharide chains identify specific cell types B. Two layers of phospholipids, the structural basis of cell membranes C. Bind extracellular substances such as hormones that trigger changes in cell activities D. Separates cell components according to their relative densities E. W ater-soluble ...
... A. Oligosaccharide chains identify specific cell types B. Two layers of phospholipids, the structural basis of cell membranes C. Bind extracellular substances such as hormones that trigger changes in cell activities D. Separates cell components according to their relative densities E. W ater-soluble ...
PP076 Allergenicity assessment strategy for novel food proteins and
... Aim: Development of an allergenicity assessment strategy for novel proteins and protein sources. Methods: Previously published literature on allergenicity risk assessment, EFSA opinions on novel foods and the use of the “weight-of-evidence approach” for food derived from GM plants were consulted. Re ...
... Aim: Development of an allergenicity assessment strategy for novel proteins and protein sources. Methods: Previously published literature on allergenicity risk assessment, EFSA opinions on novel foods and the use of the “weight-of-evidence approach” for food derived from GM plants were consulted. Re ...
ANTI- α1-SYNTROPHIN (AG-17) Developed in Rabbit, IgG Fraction
... Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or ...
... Sigma-Aldrich, Inc. warrants that its products conform to the information contained in this and other Sigma-Aldrich publications. Purchaser must determine the suitability of the product(s) for their particular use. Additional terms and conditions may apply. Please see reverse side of the invoice or ...
chapt05_lecture_anim
... less fluid than unsaturated fatty acids • “Kinks” introduced by the double bonds keep them from packing tightly • Most membranes also contain sterols such as cholesterol, which can either increase or decrease membrane fluidity, depending on the temperature ...
... less fluid than unsaturated fatty acids • “Kinks” introduced by the double bonds keep them from packing tightly • Most membranes also contain sterols such as cholesterol, which can either increase or decrease membrane fluidity, depending on the temperature ...
Binding Kinetics of Protein Lipid Interactions Using OpenSPR
... Binding Kinetics of Protein-Lipid Interactions using OpenSPR™ Procedure SUMMARY ...
... Binding Kinetics of Protein-Lipid Interactions using OpenSPR™ Procedure SUMMARY ...
Chapter 14 Proteins
... ◦ The polypeptide backbone is extended in a zigzag structure resembling a series of pleats. ◦ The six atoms of each peptide bond of a b-pleated sheet lie in the same plane. ◦ The C=O and N-H groups of the peptide bonds from adjacent chains point toward each other and are in the same plane so that hy ...
... ◦ The polypeptide backbone is extended in a zigzag structure resembling a series of pleats. ◦ The six atoms of each peptide bond of a b-pleated sheet lie in the same plane. ◦ The C=O and N-H groups of the peptide bonds from adjacent chains point toward each other and are in the same plane so that hy ...
Topic: B2b Lesson: 2 Title: Enzymes and digestion
... 2. What are the following broken down into by digestive enzymes? a) Carbohydrates simple sugars b) Proteins amino acids c) Fats fatty acids + glycerol 3. Where are most enzymes produced? Pancreas ...
... 2. What are the following broken down into by digestive enzymes? a) Carbohydrates simple sugars b) Proteins amino acids c) Fats fatty acids + glycerol 3. Where are most enzymes produced? Pancreas ...
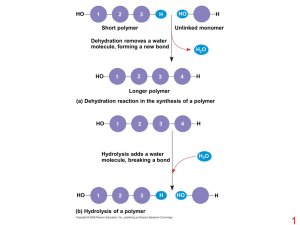
polymers - ClassNet
... shield fans from speeding pucks. Other sports uses include basketball backboards and helmet visors. PMMA is found in many common items such as acrylic paint, and various figurines, or even a chess set. Its strong and versatile, as well as fairly inexpensive, so many companies use it for toy componen ...
... shield fans from speeding pucks. Other sports uses include basketball backboards and helmet visors. PMMA is found in many common items such as acrylic paint, and various figurines, or even a chess set. Its strong and versatile, as well as fairly inexpensive, so many companies use it for toy componen ...
Macromolecules - Essentials Education
... Cytosine. The letters A, T, G and C represent these bases. A single strand of DNA is a sequence of nucleotides joined together with alternating phosphate and sugar components. The double helix molecule consists of two complementary strands that are joined by hydrogen bonds between the bases. The bas ...
... Cytosine. The letters A, T, G and C represent these bases. A single strand of DNA is a sequence of nucleotides joined together with alternating phosphate and sugar components. The double helix molecule consists of two complementary strands that are joined by hydrogen bonds between the bases. The bas ...
File
... (except soybeans and quinoa) ◦ Dried beans and peas, grains, vegetables, nuts, seeds ◦ Low in one or more essential amino acids—called the limiting amino acid. ...
... (except soybeans and quinoa) ◦ Dried beans and peas, grains, vegetables, nuts, seeds ◦ Low in one or more essential amino acids—called the limiting amino acid. ...
Chapter 11: Enzyme Catalysis
... non-polar side chain. This is because chymotrypsin's specificity pocket: A) contains a sulfhydryl group that forms a disulfide bond with the substrate. B) is lined with small hydrophobic side chains, leaving considerable room in the nonpolar pocket. C) contains a negative charge. D) is mostly filled ...
... non-polar side chain. This is because chymotrypsin's specificity pocket: A) contains a sulfhydryl group that forms a disulfide bond with the substrate. B) is lined with small hydrophobic side chains, leaving considerable room in the nonpolar pocket. C) contains a negative charge. D) is mostly filled ...
LESSON
... they are able to absorb great amounts of carbon dioxide during condensation reactions. D. they produce carbonic acid upon hydrolysis. E. All of these. ...
... they are able to absorb great amounts of carbon dioxide during condensation reactions. D. they produce carbonic acid upon hydrolysis. E. All of these. ...
Primary Structure of Proteins
... The secondary structure of a protein describes the structure that forms when amino acids form hydrogen bonds between the atoms in the backbone and atoms on the same or another peptide chain. The secondary structure in silk is a β-pleated sheet. Learning Goal Describe the primary and secondary struct ...
... The secondary structure of a protein describes the structure that forms when amino acids form hydrogen bonds between the atoms in the backbone and atoms on the same or another peptide chain. The secondary structure in silk is a β-pleated sheet. Learning Goal Describe the primary and secondary struct ...
Lecture 4: Amino Acids
... Recall that cysteine (Cys-SH HS-Cys) can convert to cystine (Cys-S-S-Cys) in the presence of air (oxidation) and will convert back if reduced. We can also prevent the formation of the disulfide bond by modifying the SH group of Cys. ...
... Recall that cysteine (Cys-SH HS-Cys) can convert to cystine (Cys-S-S-Cys) in the presence of air (oxidation) and will convert back if reduced. We can also prevent the formation of the disulfide bond by modifying the SH group of Cys. ...
The presentation part I
... Computational methods • Mentioned in this seminar, mainly for understanding proteins’ Functions and using to detect interactions ...
... Computational methods • Mentioned in this seminar, mainly for understanding proteins’ Functions and using to detect interactions ...
1333 - Protein Engineer / Structural Biologist
... Zymeworks is a fast-growing biotechnology company dedicated to the research, development and commercialization of best-in-class therapeutic bispecific antibodies and antibody drug conjugates for the treatment of cancer and autoimmune diseases. Zymeworks is seeking highly motivated scientists who are ...
... Zymeworks is a fast-growing biotechnology company dedicated to the research, development and commercialization of best-in-class therapeutic bispecific antibodies and antibody drug conjugates for the treatment of cancer and autoimmune diseases. Zymeworks is seeking highly motivated scientists who are ...
1 1 2 bez pyt lecture chemistryofaminoacids 7 fin
... - Each polypeptide chain starts on the left side by free amino group of the first amino acid enter in chain formation . It is N- terminus. - Each polypeptide chain ends on the right side by free COOH group of the last amino acid and termed (C-terminus). ...
... - Each polypeptide chain starts on the left side by free amino group of the first amino acid enter in chain formation . It is N- terminus. - Each polypeptide chain ends on the right side by free COOH group of the last amino acid and termed (C-terminus). ...
Amino Acids and Proteins
... Amino acids can undergo condensation reactions in any order, thus making it possible to form large numbers of proteins. Structurally, proteins can be described in four ways. 1. Primary 2. Secondary 3. Tertiary 4. Quaternary structure. ...
... Amino acids can undergo condensation reactions in any order, thus making it possible to form large numbers of proteins. Structurally, proteins can be described in four ways. 1. Primary 2. Secondary 3. Tertiary 4. Quaternary structure. ...
Chap. 3. "Amino Acids and the Primary Structures of Proteins
... Electrophoresis refers to the migration of charged molecules in an electric field. Molecules move toward the electrode having the opposite charge. The negatively charged electrode is called the cathode because it attracts cations. The positively charged electrode is called the anode because it attra ...
... Electrophoresis refers to the migration of charged molecules in an electric field. Molecules move toward the electrode having the opposite charge. The negatively charged electrode is called the cathode because it attracts cations. The positively charged electrode is called the anode because it attra ...
SDS-PAGE and Western blotting
... positive charges due to the charged R‐groups in the protein. The large H's represent hydrophobic domains where nonpolar R‐groups have collected in an attempt to get away from the polar water that surrounds the protein. After SDS: SDS disrupt hydrophobic areas (H's) and coat proteins with many ne ...
... positive charges due to the charged R‐groups in the protein. The large H's represent hydrophobic domains where nonpolar R‐groups have collected in an attempt to get away from the polar water that surrounds the protein. After SDS: SDS disrupt hydrophobic areas (H's) and coat proteins with many ne ...
Plasma proteins
... • Transport Iron • 2.2- 4 g/L • Synthesized in liver but affected by iron concentration in the blood • Low level leads to rise in transferrin level • Raised in anemia ...
... • Transport Iron • 2.2- 4 g/L • Synthesized in liver but affected by iron concentration in the blood • Low level leads to rise in transferrin level • Raised in anemia ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.