Plant and Soil
... using a biological oxygen monitor (Yellow Spring Instrument, Ohio), as follow. O2 consumption was measured during 5 min with 4.8 ml cell suspension (prewarmed to 25~ for 5 min) and for 10min following the addition of 200 #1 carbon substrate (to a final concentration of 10 mM organic acid or 0.5% sug ...
... using a biological oxygen monitor (Yellow Spring Instrument, Ohio), as follow. O2 consumption was measured during 5 min with 4.8 ml cell suspension (prewarmed to 25~ for 5 min) and for 10min following the addition of 200 #1 carbon substrate (to a final concentration of 10 mM organic acid or 0.5% sug ...
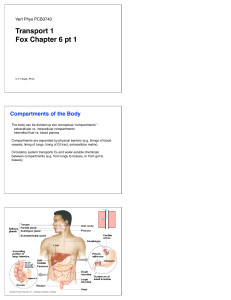
Transport 1 Fox Chapter 6 pt 1
... (different cell types express different transporters) Facilitated Diffusion Transported molecule is moved down its concentration gradient. Does not require extra energy from the cell (uses potential energy of concentration gradient). ...
... (different cell types express different transporters) Facilitated Diffusion Transported molecule is moved down its concentration gradient. Does not require extra energy from the cell (uses potential energy of concentration gradient). ...
Guided reading Ch 9- ENERGY IN A CELL
... that he is not providing any glucose for them. How is it possible that the mitochondria are still making ATP? (Think back to what an acid is, what it releases when placed in a solution and why this would make the mitochondria synthesize ATP). ...
... that he is not providing any glucose for them. How is it possible that the mitochondria are still making ATP? (Think back to what an acid is, what it releases when placed in a solution and why this would make the mitochondria synthesize ATP). ...
Supplemental notes in pdf
... (1 Calorie = 1 kilocalorie = 4.184 kilojoules), equals energy expenditure on a daily basis. Note that the relative proportions of carbohydrate, fat, and protein in our diets needs to be optimized to prevent metabolic disorders that can occur even under conditions of Caloric energy balance. For examp ...
... (1 Calorie = 1 kilocalorie = 4.184 kilojoules), equals energy expenditure on a daily basis. Note that the relative proportions of carbohydrate, fat, and protein in our diets needs to be optimized to prevent metabolic disorders that can occur even under conditions of Caloric energy balance. For examp ...
espiration - WordPress.com
... Glucose is oxidised to pyruvate during the process of glycolysis. Explain why glycolysis is said to involve oxidation. ...
... Glucose is oxidised to pyruvate during the process of glycolysis. Explain why glycolysis is said to involve oxidation. ...
Enduring Understanding: Growth, reproduction and maintenance of
... Essential Knowledge 2.A.2: Organisms capture and store free energy for use in biological processes ...
... Essential Knowledge 2.A.2: Organisms capture and store free energy for use in biological processes ...
Chlorophyll – Protein complex + H* _ OH – (Ground state)
... (ii) “storage sinks“, such as tubers, STEM, roots or fruits which deposit imported carbohydrates as storage compounds (e.g. starch, sucrose, lipid or protein). ...
... (ii) “storage sinks“, such as tubers, STEM, roots or fruits which deposit imported carbohydrates as storage compounds (e.g. starch, sucrose, lipid or protein). ...
... Glucose would be released from glycogen, so glycogen levels must fall. Glucose can also be produced from gluconeogenesis, this requires ATP, so ATP would decrease somewhat, or stay nearly constant since it can be replenished (for a while) by the formation of AMP using the following reaction: 2ADP → ...
Chemistry of Carbohydrates
... A-Starch- Polysaccharide of plant origin consist of amylose (one unbranched chain of glucose molecules linked by α-1, 4glucosidic linkages with only terminal aldehyde is free) & amylopectin (contain α-1, 4-glucosidic linkages + α-1, 6branched glucosidic linkages of glucose molecules). B-Glycogen-Po ...
... A-Starch- Polysaccharide of plant origin consist of amylose (one unbranched chain of glucose molecules linked by α-1, 4glucosidic linkages with only terminal aldehyde is free) & amylopectin (contain α-1, 4-glucosidic linkages + α-1, 6branched glucosidic linkages of glucose molecules). B-Glycogen-Po ...
File
... Glucose is split into 2 three-carbon molecules. NAD+ takes electrons, forming NADH and turning the 2 three-carbon molecules into Pyruvate. Products of Glycolysis: 1. Net gain of 2 ATP (2 “spent”, 4 made) 2. NADH 3. 2 Pyruvate ...
... Glucose is split into 2 three-carbon molecules. NAD+ takes electrons, forming NADH and turning the 2 three-carbon molecules into Pyruvate. Products of Glycolysis: 1. Net gain of 2 ATP (2 “spent”, 4 made) 2. NADH 3. 2 Pyruvate ...
12ppt - UCSD Course Websites
... every time you put an acetate in two CO2 come out before you get to OAA! ...
... every time you put an acetate in two CO2 come out before you get to OAA! ...
Advanced Cellular Respiration Worksheet
... 8. For every NADH and every FADH2 molecule oxidized in the electron transport chain of the mitochondria, how many ATP are generated (the two compounds do not result in the exact same amount of ATP produced. Your textbooks may report slightly different numbers depending on how up-to-date it is). 1 NA ...
... 8. For every NADH and every FADH2 molecule oxidized in the electron transport chain of the mitochondria, how many ATP are generated (the two compounds do not result in the exact same amount of ATP produced. Your textbooks may report slightly different numbers depending on how up-to-date it is). 1 NA ...
Krebs cycle
... Availability of oxaloacetate (OAA) is one of the main limiting steps of TCA. The [OAA] is 1/10 of the other intermediates of TCA. Remember pyruvate carboxylase is anaplerotic. Why AcetylCoA activates pyruvate carboxylase? Do you remember from glycolysis that the active metabolite (glyceraldehyde) is ...
... Availability of oxaloacetate (OAA) is one of the main limiting steps of TCA. The [OAA] is 1/10 of the other intermediates of TCA. Remember pyruvate carboxylase is anaplerotic. Why AcetylCoA activates pyruvate carboxylase? Do you remember from glycolysis that the active metabolite (glyceraldehyde) is ...
Name - chem.uwec.edu
... 39. How is the structure of cellulose different from that of amylose? b a. Cellulose has α(14) glysidic bond, but amylose has (14) glysidic bond. b. Cellulose has (14) glysidic bond, but amylose has α(14) glysidic bond. c. Cellulose has no branches, but amylose has brances. d. Cellulose has br ...
... 39. How is the structure of cellulose different from that of amylose? b a. Cellulose has α(14) glysidic bond, but amylose has (14) glysidic bond. b. Cellulose has (14) glysidic bond, but amylose has α(14) glysidic bond. c. Cellulose has no branches, but amylose has brances. d. Cellulose has br ...
Cellular Respiration
... into two molecules of the three-carbon compound pyruvate (pyruvic acid) occurs in all cells gives a net gain of 2 ATP molecules in anaerobic respiration it is the only stage of respiration ...
... into two molecules of the three-carbon compound pyruvate (pyruvic acid) occurs in all cells gives a net gain of 2 ATP molecules in anaerobic respiration it is the only stage of respiration ...
Insulina - Gilberto De Nucci
... hypoglycemic events noted per patient were reduced by 46.4% (p = 0.003) and occurred significantly less often during nocturnal periods (-63.2%, p = 0.002). Following the adjustment, the mean daily insulin requirement was reduced by 28.05% (from 0.82 to 0.59 IU/kg) and the new proportion of 40% as ba ...
... hypoglycemic events noted per patient were reduced by 46.4% (p = 0.003) and occurred significantly less often during nocturnal periods (-63.2%, p = 0.002). Following the adjustment, the mean daily insulin requirement was reduced by 28.05% (from 0.82 to 0.59 IU/kg) and the new proportion of 40% as ba ...
2 Pyruvic Acid
... During respiration electrons are removed from glucose and transported to the ETC by electron carriers. Energy from the electrons is used to synthesize ATP in the ETC. ...
... During respiration electrons are removed from glucose and transported to the ETC by electron carriers. Energy from the electrons is used to synthesize ATP in the ETC. ...
labmuscle
... acid plays an important role in generating energy physical endurance to help one survive. It is used to as fuel during exercise and recovery. The process in which lactic acid is formed is called anaerobic metabolism because it does not use oxygen. During this process, the body breaks down carbohydra ...
... acid plays an important role in generating energy physical endurance to help one survive. It is used to as fuel during exercise and recovery. The process in which lactic acid is formed is called anaerobic metabolism because it does not use oxygen. During this process, the body breaks down carbohydra ...
intermediary metabolism
... Cell metabolism operates at maximum economy. The overall rate of energy yielding catabolism is controlled by the needs of the cell for energy in the form of ATP and NADPH. Thus cells conserve just enough nutrients to meet the energy utilization at any given time. Similarly the rate of biosynthesis o ...
... Cell metabolism operates at maximum economy. The overall rate of energy yielding catabolism is controlled by the needs of the cell for energy in the form of ATP and NADPH. Thus cells conserve just enough nutrients to meet the energy utilization at any given time. Similarly the rate of biosynthesis o ...
Document
... + 2 ATP + 2 NADH + 2 H+ (cytoplasm) + 6CO2 + 8 NADH + 8 H+ + 2 FADH2 + 2 GTP (mitochondria) ...
... + 2 ATP + 2 NADH + 2 H+ (cytoplasm) + 6CO2 + 8 NADH + 8 H+ + 2 FADH2 + 2 GTP (mitochondria) ...
Chapter 9 Notes
... degradation of sugars or other organic fuel that occurs without the use of oxygen. The most prevalent and efficient catabolic pathway is aerobic respiration. -oxygen is consumed as a reactant along with the organic fuel. The cells of most eukaryotic and many prokaryotic organisms can carry out aerob ...
... degradation of sugars or other organic fuel that occurs without the use of oxygen. The most prevalent and efficient catabolic pathway is aerobic respiration. -oxygen is consumed as a reactant along with the organic fuel. The cells of most eukaryotic and many prokaryotic organisms can carry out aerob ...
Glucose

Glucose is a sugar with the molecular formula C6H12O6. The name ""glucose"" (/ˈɡluːkoʊs/) comes from the Greek word γλευκος, meaning ""sweet wine, must"". The suffix ""-ose"" is a chemical classifier, denoting a carbohydrate. It is also known as dextrose or grape sugar. With 6 carbon atoms, it is classed as a hexose, a sub-category of monosaccharides. α-D-glucose is one of the 16 aldose stereoisomers. The D-isomer (D-glucose) occurs widely in nature, but the L-isomer (L-glucose) does not. Glucose is made during photosynthesis from water and carbon dioxide, using energy from sunlight. The reverse of the photosynthesis reaction, which releases this energy, is a very important source of power for cellular respiration. Glucose is stored as a polymer, in plants as starch and in animals as glycogen.