PHYS 203 General Physics
... total energy is three times that, or 1.53 MeV? 9. Suppose that Planck’s constant h were to change from 6.6×10−34 J-s to 0.066 J-s. What kind of effects might you notice from this change? ...
... total energy is three times that, or 1.53 MeV? 9. Suppose that Planck’s constant h were to change from 6.6×10−34 J-s to 0.066 J-s. What kind of effects might you notice from this change? ...
Quantum Mechanical Model
... You may want to find electrons within the sublevels..but remember: The Quantum Theory describes “probability clouds”; these are called atomic orbitals. Atomic Orbitals are designated by letters. (Draw models) (w/periodic table) Principle Energy Level ...
... You may want to find electrons within the sublevels..but remember: The Quantum Theory describes “probability clouds”; these are called atomic orbitals. Atomic Orbitals are designated by letters. (Draw models) (w/periodic table) Principle Energy Level ...
Unit 4 Study Guide - Key - Effingham County Schools
... 5. _Wavelength____________ (λ) is the distance between corresponding points on adjacent waves. What is its unit? _m, cm, or nm_________ 6. _Frequency____________ (ν) is defined as the number of waves that pass a given point in a specific time. What is its unit? _waves/second or hertz(Hz)___________ ...
... 5. _Wavelength____________ (λ) is the distance between corresponding points on adjacent waves. What is its unit? _m, cm, or nm_________ 6. _Frequency____________ (ν) is defined as the number of waves that pass a given point in a specific time. What is its unit? _waves/second or hertz(Hz)___________ ...
Periodic Table Jeopardy
... substances by chemical means. All atoms in this substance have the same atomic #. ...
... substances by chemical means. All atoms in this substance have the same atomic #. ...
Fermi-Dirac Statistics
... A two-dimensional model provides a useful means to compute the integral for U(T) graphically, since D(E)=constant in 2D. We consider that all electrons within kBT of EF have their energy increased by exactly kBT . All other electrons do not change their energy, as their occupancy remains unity. Thus ...
... A two-dimensional model provides a useful means to compute the integral for U(T) graphically, since D(E)=constant in 2D. We consider that all electrons within kBT of EF have their energy increased by exactly kBT . All other electrons do not change their energy, as their occupancy remains unity. Thus ...
εn = ε KE + ε PE = ε PE ε PE = ε PE (1 )
... REPULSION which will occur when 2 electrons are placed in the SAME SPATIAL ORBITAL -- such REPULSION would RAISE the energy of the atom This result is summarised in HUND'S RULE: Other things being equal, THE STATE OF LOWEST ENERGY corresponds to the MAXIMUM NUMBER OF UNPAIRED, PARALLEL SPINS Thus th ...
... REPULSION which will occur when 2 electrons are placed in the SAME SPATIAL ORBITAL -- such REPULSION would RAISE the energy of the atom This result is summarised in HUND'S RULE: Other things being equal, THE STATE OF LOWEST ENERGY corresponds to the MAXIMUM NUMBER OF UNPAIRED, PARALLEL SPINS Thus th ...
Chapter 2 Part 1 ppt
... Electrons - energy can be measured very accurately, therefore cannot know position (x) with any certainty • Probability of finding an electron at any position (electron density = probability) • Both Schrodinger and Heisenberg proposed ways to treat electrons as waves, Schrodinger’s math was easier ...
... Electrons - energy can be measured very accurately, therefore cannot know position (x) with any certainty • Probability of finding an electron at any position (electron density = probability) • Both Schrodinger and Heisenberg proposed ways to treat electrons as waves, Schrodinger’s math was easier ...
Chapter 5 reveiw
... 14. Any orbital, no matter what sublevel (s, p, d, f) it is in, can hold up to 2 electrons. 15. The maximum no. of electrons in a sublevel is (s=2, p = 6, d = 10, f= 14) 16. The number of orbitals in each sublevel is s = 1, p = 3, d = 5, f = 7 a. The maximum number of electrons in the each principal ...
... 14. Any orbital, no matter what sublevel (s, p, d, f) it is in, can hold up to 2 electrons. 15. The maximum no. of electrons in a sublevel is (s=2, p = 6, d = 10, f= 14) 16. The number of orbitals in each sublevel is s = 1, p = 3, d = 5, f = 7 a. The maximum number of electrons in the each principal ...
Atomic Structure and Quantum Theory Photon Energies
... state to an excited state. • Energy is released (often as light) when an electron drops from an excited state to the ground state. ...
... state to an excited state. • Energy is released (often as light) when an electron drops from an excited state to the ground state. ...
27-3 A Photoelectric Effect Example
... Using the experimental apparatus shown in Figure 27.5, when ultraviolet light with a wavelength of 240 nm shines on a particular metal plate, electrons are emitted from plate 1, crossing the gap to plate 2 and causing a current to flow through the wire connecting the two plates. The battery voltage ...
... Using the experimental apparatus shown in Figure 27.5, when ultraviolet light with a wavelength of 240 nm shines on a particular metal plate, electrons are emitted from plate 1, crossing the gap to plate 2 and causing a current to flow through the wire connecting the two plates. The battery voltage ...
Unit 4 review sheet
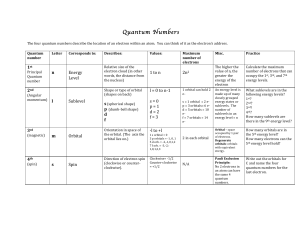
... 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What four letters are used to represent the sublevels within a principle energy level? 40. What is the maximum number of electrons that may occupy one p orbital? 41. Who stated that ...
... 37. How many quantum numbers are there? 38. What letter denotes the quantum number for the principle energy level? 39. What four letters are used to represent the sublevels within a principle energy level? 40. What is the maximum number of electrons that may occupy one p orbital? 41. Who stated that ...
2013.9.23
... Si Conduction-Band Structure in wave vector k-space (Constant-Energy Surfaces in k-space)Effective mass approximation: Kinetic energy ...
... Si Conduction-Band Structure in wave vector k-space (Constant-Energy Surfaces in k-space)Effective mass approximation: Kinetic energy ...
Spectral Lines - Transcript
... An electron in the lowest energy shell in an atom can be struck by and absorb the energy of a photon giving it enough energy to jump to the next energy shell. And the reverse process allows the electron to jump back down into the lowest energy shell and emit a photon. The color of the photon depends ...
... An electron in the lowest energy shell in an atom can be struck by and absorb the energy of a photon giving it enough energy to jump to the next energy shell. And the reverse process allows the electron to jump back down into the lowest energy shell and emit a photon. The color of the photon depends ...
ap chemistry review – multiple choice
... 3. The energy in a chemical or physical change that is available to do useful work, 4. The energy required to form the transition state in a chemical reaction. Questions 5-8 refer to atoms for which the occupied atomic orbitals are shown below: (a) 1s ___ (b) 1s (c) 1s (d) 1s (e) [Ar] 4s ...
... 3. The energy in a chemical or physical change that is available to do useful work, 4. The energy required to form the transition state in a chemical reaction. Questions 5-8 refer to atoms for which the occupied atomic orbitals are shown below: (a) 1s ___ (b) 1s (c) 1s (d) 1s (e) [Ar] 4s ...
1. Define the vocabulary on page 88. Section 1
... 3. All forms of electromagnetic radiation move at a constant speed of _____________ through a vacuum. 4. _________ is the distance between corresponding points on adjacent waves. 5. What is the symbol for wavelength? 6. Frequency is defined as _______________________________________. 7. What is the ...
... 3. All forms of electromagnetic radiation move at a constant speed of _____________ through a vacuum. 4. _________ is the distance between corresponding points on adjacent waves. 5. What is the symbol for wavelength? 6. Frequency is defined as _______________________________________. 7. What is the ...
WS on obj. 1-11
... 23. _____ (T/F) Cations are formed by the gain of protons. 24. ____________________________ ions are the ions of the halogens and have a 1- charge, 25. _____________________________________ compounds are composed of positive and negative ions. 26. _____ (T/F) A formula unit shows the smallest whole- ...
... 23. _____ (T/F) Cations are formed by the gain of protons. 24. ____________________________ ions are the ions of the halogens and have a 1- charge, 25. _____________________________________ compounds are composed of positive and negative ions. 26. _____ (T/F) A formula unit shows the smallest whole- ...
Quantum Mechanics Physics
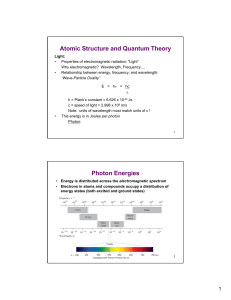
... • Atomic Spectroscopy is the analytical measurement of the quantum energy level jumps of different electron energy states. • It is a spectral analysis of the colors (frequencies or wavelengths) that an atom gives off when it changes energy levels. • Formulas: c = f and 1/ = R(1/nf2 –1/ni2) where ...
... • Atomic Spectroscopy is the analytical measurement of the quantum energy level jumps of different electron energy states. • It is a spectral analysis of the colors (frequencies or wavelengths) that an atom gives off when it changes energy levels. • Formulas: c = f and 1/ = R(1/nf2 –1/ni2) where ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.