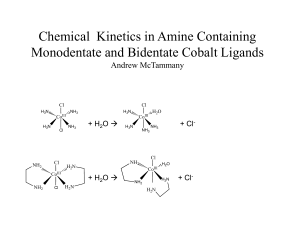
Kinetic Modeling Of Methanol Synthesis From Carbon Monoxide
... using supports and promoters [2, 3]. Major kinetic studies for methanol synthesis were done as early as 1977, and, even recently, authors are trying to model the process kinetics [2]. Although reaction mechanisms for this process have been studied for decades now, there has been no agreement on one ...
... using supports and promoters [2, 3]. Major kinetic studies for methanol synthesis were done as early as 1977, and, even recently, authors are trying to model the process kinetics [2]. Although reaction mechanisms for this process have been studied for decades now, there has been no agreement on one ...
Kinetic multi-layer model of aerosol surface and bulk chemistry (KM
... we show how interfacial transport and bulk transport, i.e., surface accommodation, bulk accommodation and bulk diffusion, influence the kinetics of the chemical reaction. Sensitivity studies suggest that in fine air particulate matter oleic acid and compounds with similar reactivity against ozone (C ...
... we show how interfacial transport and bulk transport, i.e., surface accommodation, bulk accommodation and bulk diffusion, influence the kinetics of the chemical reaction. Sensitivity studies suggest that in fine air particulate matter oleic acid and compounds with similar reactivity against ozone (C ...
UNIVERSITY OF TARTU THE GIFTED AND
... 2. According to Faraday’s first law the amount of substance generated on electrodes during electrolysis is proportional to the charge conducted by the electrodes. The Faraday constant (F= 96 500 C/mol) shows the charge of one mole of molecules. During the electrolysis of Na2SO4, a total charge of 23 ...
... 2. According to Faraday’s first law the amount of substance generated on electrodes during electrolysis is proportional to the charge conducted by the electrodes. The Faraday constant (F= 96 500 C/mol) shows the charge of one mole of molecules. During the electrolysis of Na2SO4, a total charge of 23 ...
Chapter 12: Chemical Equilibrium • Chemical Equilibrium
... decrease in rate of the forward reaction. • As the reactants are being consumed, the product concentration increases, with a corresponding increase in the rate of the reverse reaction. • When the rate of the forward reaction equals the rate of the reverse reaction, the reaction has reached equilibri ...
... decrease in rate of the forward reaction. • As the reactants are being consumed, the product concentration increases, with a corresponding increase in the rate of the reverse reaction. • When the rate of the forward reaction equals the rate of the reverse reaction, the reaction has reached equilibri ...
chemistry - Rwanda Education Board
... involved into the teaching and learning process. Good moral values can be cultivated through suitable contexts. Learning in groups should be emphasized to help learners to develop social skills, encourage cooperation and build self confidence. Environment awareness and conservation skills should als ...
... involved into the teaching and learning process. Good moral values can be cultivated through suitable contexts. Learning in groups should be emphasized to help learners to develop social skills, encourage cooperation and build self confidence. Environment awareness and conservation skills should als ...