Chapter 9 Quantum Mechanics
... called Quantum Mechanics, which is valid in a very small region but its large scale derivation could also give classical results. It is known that quantum theory is a very important theory not only in physics, but also in many branches of science, such as life science and medical science. The concep ...
... called Quantum Mechanics, which is valid in a very small region but its large scale derivation could also give classical results. It is known that quantum theory is a very important theory not only in physics, but also in many branches of science, such as life science and medical science. The concep ...
Music and harmonics - BYU Physics and Astronomy
... • Some older treatments of relativity maintained the conservation of momentum principle at high speeds by using a model in which the mass of the particle increases with speed. You might still encounter this notion of “relativistic mass” in your outside reading, especially in older books. Be aware th ...
... • Some older treatments of relativity maintained the conservation of momentum principle at high speeds by using a model in which the mass of the particle increases with speed. You might still encounter this notion of “relativistic mass” in your outside reading, especially in older books. Be aware th ...
Fulltext
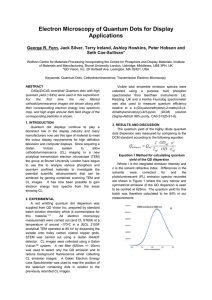
... quantum yield (~84%) were used in this experiment. For the first time the red filtered cathodoluminescence images are shown along with their corresponding electron energy loss spectrum map, and high angle annular dark field image of the corresponding particles is shown. 1. INTRODUCTION Quantum dot d ...
... quantum yield (~84%) were used in this experiment. For the first time the red filtered cathodoluminescence images are shown along with their corresponding electron energy loss spectrum map, and high angle annular dark field image of the corresponding particles is shown. 1. INTRODUCTION Quantum dot d ...
Neils Bohr
... energy orbit to a lower orbit. Also showed the light quantum of distinct energy, which formed the bases for the quantum theory. - Quantum theory showed the specific relation proportion of an electron and how it circulates the atoms’ nucleus ...
... energy orbit to a lower orbit. Also showed the light quantum of distinct energy, which formed the bases for the quantum theory. - Quantum theory showed the specific relation proportion of an electron and how it circulates the atoms’ nucleus ...
spectral lines
... Bohr to the rescue In 1905, Einstein had proposed the wave/particle duality of light with the photon. In 1913, Bohr used the concept in creating a quantum model of the atom. ...
... Bohr to the rescue In 1905, Einstein had proposed the wave/particle duality of light with the photon. In 1913, Bohr used the concept in creating a quantum model of the atom. ...
Particle wavelength, Rutherford scattering
... shown here on a bacterium). This picture is the output of an “electron microscope” ...
... shown here on a bacterium). This picture is the output of an “electron microscope” ...
450 AD and Prior Democritus - reich
... Anode rays were observed in experiments by a German scientist, Eugen Goldstein, in 1886. Goldstein used a gas discharge tube which had a perforated cathode. A "ray" is produced in the holes (canals) in the cathode and travels in a direction opposite to the "cathode rays," which are streams of electr ...
... Anode rays were observed in experiments by a German scientist, Eugen Goldstein, in 1886. Goldstein used a gas discharge tube which had a perforated cathode. A "ray" is produced in the holes (canals) in the cathode and travels in a direction opposite to the "cathode rays," which are streams of electr ...
The Heisenberg Uncertainty Principle
... The Heisenberg Uncertainty Principle The Heisenberg uncertainty principle states that it is impossible to know both the momentum and the position of a particle at the same time. This limitation is critical when dealing with small particles such as electrons. But it does not matter for ordinary- ...
... The Heisenberg Uncertainty Principle The Heisenberg uncertainty principle states that it is impossible to know both the momentum and the position of a particle at the same time. This limitation is critical when dealing with small particles such as electrons. But it does not matter for ordinary- ...
Symmetry and Its Violation -unifying concept of universe
... anti-quarks and (W, Z0) anti-leptons strong (gluon: g) The Standard Model ...
... anti-quarks and (W, Z0) anti-leptons strong (gluon: g) The Standard Model ...
what`s ahead in particle physics - CMS DocDB Server
... according to some of the most interesting theories of dark matter, the dark matter particles are also lurking at the Terascale. ...
... according to some of the most interesting theories of dark matter, the dark matter particles are also lurking at the Terascale. ...
summary 2015
... have lifetimes of 2 s, so should travel only a few hundred metres before decaying. Their speeds are close to c, so most of them travel through many tens of km of atmosphere due to ...
... have lifetimes of 2 s, so should travel only a few hundred metres before decaying. Their speeds are close to c, so most of them travel through many tens of km of atmosphere due to ...
ppt - HEP Educational Outreach
... Build inquiry-based learning environment … researchers build knowledge through inquiry, discussion, collaboration ...
... Build inquiry-based learning environment … researchers build knowledge through inquiry, discussion, collaboration ...
Atoms and Energies
... Light exhibits wavelike properties when traveling Light exhibits particlelike properties when interacting with matter deBroglie suggested that traditional “particles”, like the electron, also exhibit wavelike properties p=h/l, so large (macroscopic) momentum means small (undetectable) wavelength ...
... Light exhibits wavelike properties when traveling Light exhibits particlelike properties when interacting with matter deBroglie suggested that traditional “particles”, like the electron, also exhibit wavelike properties p=h/l, so large (macroscopic) momentum means small (undetectable) wavelength ...
subatomic-particles
... and electrons are subatomic particles. Did You Know? Until the 20th century, scientists thought that atoms were the smallest units of matter. This view started to change following some experiments in the late 1800s involving electrical discharges. By the early 1900s it was clear that these discharge ...
... and electrons are subatomic particles. Did You Know? Until the 20th century, scientists thought that atoms were the smallest units of matter. This view started to change following some experiments in the late 1800s involving electrical discharges. By the early 1900s it was clear that these discharge ...