Topic 22 Notes
... H3C C CH3 ALKANE A. Definition An alkane is a hydrocarbon containing only carbon-carbon single bonds B. Can be divided into three categories based on shape 1. Straight-chain – all carbons connected in a row 2. Branched chain – have branching connections of carbons 3. Cyclic – have three or more carb ...
... H3C C CH3 ALKANE A. Definition An alkane is a hydrocarbon containing only carbon-carbon single bonds B. Can be divided into three categories based on shape 1. Straight-chain – all carbons connected in a row 2. Branched chain – have branching connections of carbons 3. Cyclic – have three or more carb ...
stoker-C15
... (1) The compound 4-oxopentanal contains both an aldehyde and an ether functional group. (2) An alkoxy group and a hydroxy group attached to the same carbon atom are present in both hemiacetals and acetals. (3) Cyclic aldehyde structures are possible but cyclic ketone structures are not possible. A) ...
... (1) The compound 4-oxopentanal contains both an aldehyde and an ether functional group. (2) An alkoxy group and a hydroxy group attached to the same carbon atom are present in both hemiacetals and acetals. (3) Cyclic aldehyde structures are possible but cyclic ketone structures are not possible. A) ...
organic practice problems
... ____ 57. All of the statements below concerning hydrocarbons are correct EXCEPT a. most are insoluble in water. b. most have only covalent bonding. c. most are gases at room temperature. d. they tend to be oxidized by O 2 to form CO2 and H2O. e. their solubility in nonpolar solvents are higher than ...
... ____ 57. All of the statements below concerning hydrocarbons are correct EXCEPT a. most are insoluble in water. b. most have only covalent bonding. c. most are gases at room temperature. d. they tend to be oxidized by O 2 to form CO2 and H2O. e. their solubility in nonpolar solvents are higher than ...
Organic Chemistry for Highschool Biology students
... 5. Very often, a molecule can be considered to be part of many families because they have more than one functional group. Odor 11 is a good example of this situation. ...
... 5. Very often, a molecule can be considered to be part of many families because they have more than one functional group. Odor 11 is a good example of this situation. ...
Organic Chemistry: Introduction
... • related compounds that have the same functional group (groups of atoms found within molecules that are involved in the chemical reactions characteristic of those molecules) ...
... • related compounds that have the same functional group (groups of atoms found within molecules that are involved in the chemical reactions characteristic of those molecules) ...
10 Haloalkanes and Haloarenes
... the addition of HX in unsymmetrical alkene takes place according to Markownikoff’s rule. According to Markownikoff’s rule, “during addition of an unsymmetrical reagent across the double bond of an unsymmetrical alkene, the negative part of reagent attacks on the carbon atom with less number of hydro ...
... the addition of HX in unsymmetrical alkene takes place according to Markownikoff’s rule. According to Markownikoff’s rule, “during addition of an unsymmetrical reagent across the double bond of an unsymmetrical alkene, the negative part of reagent attacks on the carbon atom with less number of hydro ...
Topic 10.1 Fundametals of Organic Chemistry
... – not accepted in the IB for answers but often used in questions – every “corner” represents a carbon – hydrogens are implied ...
... – not accepted in the IB for answers but often used in questions – every “corner” represents a carbon – hydrogens are implied ...
Organic Chemistry
... Only van der Waals force: London force. Boiling point increases with length of chain. Combust to give mainly CO2 and H2O Nomenclature suffix “‐ane” ...
... Only van der Waals force: London force. Boiling point increases with length of chain. Combust to give mainly CO2 and H2O Nomenclature suffix “‐ane” ...
Nomenclature Chapter
... R = any general carbon group (it sometimes includes hydrogen too) Ar = any general aromatic group, (when more specificity than ‘just’ R is desired) The foundation of organic nomenclature requires an ability to name alkanes, alkenes and alkynes. Learning the rules for these groups will be your bigges ...
... R = any general carbon group (it sometimes includes hydrogen too) Ar = any general aromatic group, (when more specificity than ‘just’ R is desired) The foundation of organic nomenclature requires an ability to name alkanes, alkenes and alkynes. Learning the rules for these groups will be your bigges ...
Unit 2
... then passes into a fractionating _ _ _ _ _ _ and begins its journey upwards from the hottest region at the bottom towards the coolest region at the top. Hydrocarbons with similar boiling _ _ _ _ _ _ condense at different _ _ _ _ _ _ and flow into collecting trays. Each so called _ _ _ _ _ _ _ _ cont ...
... then passes into a fractionating _ _ _ _ _ _ and begins its journey upwards from the hottest region at the bottom towards the coolest region at the top. Hydrocarbons with similar boiling _ _ _ _ _ _ condense at different _ _ _ _ _ _ and flow into collecting trays. Each so called _ _ _ _ _ _ _ _ cont ...
CHM 103 Lecture 23 S07
... groups (R(R-O-R’). • has a common name that gives the alkyl names of the attached groups followed by ether. ether. CH3─O─CH3 CH3─CH2─O─CH3 ...
... groups (R(R-O-R’). • has a common name that gives the alkyl names of the attached groups followed by ether. ether. CH3─O─CH3 CH3─CH2─O─CH3 ...
Organic Chemistry introduction
... MP Branching reduces the flexibility of the molecule which reduces the entropy term S in the equation Tmp = H/S. Since S is in the denominator, Tmp increases. BP Branching reduces surface area (more compact structure), and therefore London dispersion forces which control boiling point for these ...
... MP Branching reduces the flexibility of the molecule which reduces the entropy term S in the equation Tmp = H/S. Since S is in the denominator, Tmp increases. BP Branching reduces surface area (more compact structure), and therefore London dispersion forces which control boiling point for these ...
Stereochemistry Tutorials: Assigning R/S and E/Z
... To provide the full name for the molecule, R or S is added in parenthesis in front of the name. Thus, this molecule is (R)-2-chlorobutane. If there is more than one stereocenter, each R or S is numbered with its position on the molecular skeleton. Example: (2R,3S)-2,3-butanediol. HO ...
... To provide the full name for the molecule, R or S is added in parenthesis in front of the name. Thus, this molecule is (R)-2-chlorobutane. If there is more than one stereocenter, each R or S is numbered with its position on the molecular skeleton. Example: (2R,3S)-2,3-butanediol. HO ...
Stereochemistry Tutorials: Assigning R/S and E/Z
... To provide the full name for the molecule, R or S is added in parenthesis in front of the name. Thus, this molecule is (R)-2-chlorobutane. If there is more than one stereocenter, each R or S is numbered with its position on the molecular skeleton. Example: (2R,3S)-2,3-butanediol. HO ...
... To provide the full name for the molecule, R or S is added in parenthesis in front of the name. Thus, this molecule is (R)-2-chlorobutane. If there is more than one stereocenter, each R or S is numbered with its position on the molecular skeleton. Example: (2R,3S)-2,3-butanediol. HO ...
Unit 5: Oragnic Chemistry Notes (answers)
... any effect on their physical properties. 3. They do give off more heat (more exothermic) when burned (combusted) compare to alkanes. This is because there are more energy stored in the double and triple bonds. When this energy is released during a chemical reaction, more heat is given off. Combustio ...
... any effect on their physical properties. 3. They do give off more heat (more exothermic) when burned (combusted) compare to alkanes. This is because there are more energy stored in the double and triple bonds. When this energy is released during a chemical reaction, more heat is given off. Combustio ...
Unit 13: Organic Chemistry
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
Unit 13: Organic Chemistry
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
Unit 13: Organic Chemistry
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
... (H) bonded to a primary chain carbon. 4. Alkane: A hydrocarbon with the general formula CnH2n+2, where all the carboncarbon bonds are single bonds. 5. Alkene: A hydrocarbon with the general formula CnH2n, where only one of the carbon-carbon bonds is a double bond. 6. Alkyl Group: An alkane fragment ...
Organic Halides (Haloalkanes) (Alkyl Halides)
... Prefixes for halides are inserted into the name of the structure in the exact same fashion as any alkyl group. Remember to write them in alphabetical order ...
... Prefixes for halides are inserted into the name of the structure in the exact same fashion as any alkyl group. Remember to write them in alphabetical order ...
ETHER
... involves carbon. Such compounds are called ethers. For example, dimethyl ether is isomeric with ethanol, and methyl ethyl ether is isomeric with propanol. Crown Ethers: These cyclic polyethers can form chemical complexes with metal cations (such as lithium, sodium, and potassium cations), thus holdi ...
... involves carbon. Such compounds are called ethers. For example, dimethyl ether is isomeric with ethanol, and methyl ethyl ether is isomeric with propanol. Crown Ethers: These cyclic polyethers can form chemical complexes with metal cations (such as lithium, sodium, and potassium cations), thus holdi ...
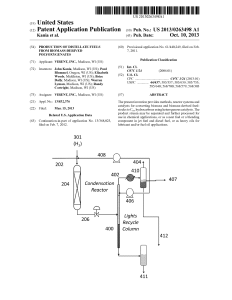
Production of Distillate Fuels from Biomass
... oped to overcome these issues and provide liquid fuels and chemicals derived from the cellulose, hemicellulose and lig nin found in plant cell Walls. For instance, cellulose and hemicellulose canbe used as feedstock for various bioreform ...
... oped to overcome these issues and provide liquid fuels and chemicals derived from the cellulose, hemicellulose and lig nin found in plant cell Walls. For instance, cellulose and hemicellulose canbe used as feedstock for various bioreform ...
Chapter 12, Alkenes and Alkynes
... Alkynes are hydrocarbons which have one or more carbon-carbon triple bonds. These have little import to biochemistry and will not be studied further in this course. Fig. 12.UN, p.314 ...
... Alkynes are hydrocarbons which have one or more carbon-carbon triple bonds. These have little import to biochemistry and will not be studied further in this course. Fig. 12.UN, p.314 ...
Alkane
In organic chemistry, an alkane, or paraffin (a historical name that also has other meanings), is a saturated hydrocarbon. Alkanes consist only of hydrogen and carbon atoms and all bonds are single bonds. Alkanes (technically, always acyclic or open-chain compounds) have the general chemical formula CnH2n+2. For example, Methane is CH4, in which n=1 (n being the number of Carbon atoms). Alkanes belong to a homologous series of organic compounds in which the members differ by a molecular mass of 14.03u (mass of a methanediyl group, —CH2—, one carbon atom of mass 12.01u, and two hydrogen atoms of mass ≈1.01u each). There are two main commercial sources: petroleum (crude oil) and natural gas.Each carbon atom has 4 bonds (either C-H or C-C bonds), and each hydrogen atom is joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. The number of carbon atoms is used to define the size of the alkane e.g., C2-alkane.An alkyl group, generally abbreviated with the symbol R, is a functional group or side-chain that, like an alkane, consists solely of single-bonded carbon and hydrogen atoms, for example a methyl or ethyl group.The simplest possible alkane (the parent molecule) is methane, CH4. There is no limit to the number of carbon atoms that can be linked together, the only limitation being that the molecule is acyclic, is saturated, and is a hydrocarbon. Waxes include examples of larger alkanes where the number of carbons in the carbon backbone is greater than about 17, above which the compounds are solids at standard ambient temperature and pressure (SATP).Alkanes are not very reactive and have little biological activity. All alkanes are colourless and odourless. Alkanes can be viewed as a molecular tree upon which can be hung the more biologically active/reactive portions (functional groups) of the molecule.