thermdyn - chemmybear.com
... the standard heat of formation of solid sodium 1985 D chloride. Indicate whether each factor makes the (a) When liquid water is introduced into an evacuated reaction for the formation of sodium chloride from vessel at 25C, some of the water vaporizes. Preits elements more or less exothermic. dict h ...
... the standard heat of formation of solid sodium 1985 D chloride. Indicate whether each factor makes the (a) When liquid water is introduced into an evacuated reaction for the formation of sodium chloride from vessel at 25C, some of the water vaporizes. Preits elements more or less exothermic. dict h ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... • This reaction gives the predicted product, but you better carry it out in the hood, or you will be very unpopular! • Just as in the previous examples, a gas is formed as a product of this reaction: Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Aqueous Reactions ...
... • This reaction gives the predicted product, but you better carry it out in the hood, or you will be very unpopular! • Just as in the previous examples, a gas is formed as a product of this reaction: Na2S (aq) + H2SO4 (aq) Na2SO4 (aq) + H2S (g) Aqueous Reactions ...
Chemical Equations and Reactions
... To know for certain that a chemical reaction has taken place requires evidence that one or more substances have undergone a change in identity. Absolute proof of such a change can be provided only by chemical analysis of the products. However, certain easily observed changes usually indicate that a ...
... To know for certain that a chemical reaction has taken place requires evidence that one or more substances have undergone a change in identity. Absolute proof of such a change can be provided only by chemical analysis of the products. However, certain easily observed changes usually indicate that a ...
+ ∂ - CHEM171 – Lecture Series Seven : 2012/05
... A hydrocarbon of unknown structure has the molecular formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a Pd/C catalyst 3 equivalents of H2 react. Draw a structure for the hydrocarbon that fits the data. ...
... A hydrocarbon of unknown structure has the molecular formula C8H10. On catalytic hydrogenation over the Lindlar catalyst, 1 equivalent of H2 is absorbed. On hydrogenation over a Pd/C catalyst 3 equivalents of H2 react. Draw a structure for the hydrocarbon that fits the data. ...
Molecular orbital approach to substituent effects in amine
... and detailed molecular orbital calculations using the M N D O method.' Experiments that support the results obtained by molecular orbital theory are also described. The understanding of the effects of substituents on the donor properties of amino species resulting from these studies is then related ...
... and detailed molecular orbital calculations using the M N D O method.' Experiments that support the results obtained by molecular orbital theory are also described. The understanding of the effects of substituents on the donor properties of amino species resulting from these studies is then related ...
108B Carbohydrate Activity KEY
... 4. Monosaccharides can act as nucleophiles and/or electrophiles. Redraw any sugar from #1d and #1e and indicate the functional groups that could act as nucleophiles and those that can serve as electrophiles. alcohol = nucleophile aldehyde = electrophile CHO H OH H OH 4 alcohols = nucleophiles HO H C ...
... 4. Monosaccharides can act as nucleophiles and/or electrophiles. Redraw any sugar from #1d and #1e and indicate the functional groups that could act as nucleophiles and those that can serve as electrophiles. alcohol = nucleophile aldehyde = electrophile CHO H OH H OH 4 alcohols = nucleophiles HO H C ...
2007 Final Exam - Oregon State chemistry
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your last name, first name, middle init ...
... calculator, and your University ID Card. If you have notes with you, place them in a sealed backpack and place the backpack OUT OF SIGHT or place the notes directly on the table at the front of the room. Fill in the front page of the Scantron answer sheet with your last name, first name, middle init ...
Year 2 Chemistry Contents Guide
... • Introduction to acid–base titration Animations illustrating pH curves for the following titrations: strong acid vs. strong base, weak acid vs. strong base, strong acid vs. weak base and weak acid vs. weak base Example worked calculations from the results of titration experiments • Selecting an app ...
... • Introduction to acid–base titration Animations illustrating pH curves for the following titrations: strong acid vs. strong base, weak acid vs. strong base, strong acid vs. weak base and weak acid vs. weak base Example worked calculations from the results of titration experiments • Selecting an app ...
Chemical Reactions
... hazardous compounds. The Hamilton fire was an example of chemical reactions allowed to proceed in an uncontrolled fashion. The fire was also an example of the release of energy that accompanies many chemical reactions. Energy, whether produced or absorbed, is an important aspect of chemical reaction ...
... hazardous compounds. The Hamilton fire was an example of chemical reactions allowed to proceed in an uncontrolled fashion. The fire was also an example of the release of energy that accompanies many chemical reactions. Energy, whether produced or absorbed, is an important aspect of chemical reaction ...
esterification of palmitic acid with methanol in the
... The esterification of palmitic acid with methanol was carried out in a batch reactor system. The data were obtained to study the kinetics of the reaction and to evaluate the kinetic parameters of the esterification process. A pre-mixed method was opted in order to study the behavior of the heterogen ...
... The esterification of palmitic acid with methanol was carried out in a batch reactor system. The data were obtained to study the kinetics of the reaction and to evaluate the kinetic parameters of the esterification process. A pre-mixed method was opted in order to study the behavior of the heterogen ...
H2 Chemistry Syllabus (9729)
... matter and energy in the quantitative treatment of reactions such as stoichiometry and thermochemistry. At A Level, an in-depth study of the electronic structure of atoms provides the basis for the study of chemical bonding. The Valence Shell Electron Pair Repulsion (VSEPR) model is used to visualis ...
... matter and energy in the quantitative treatment of reactions such as stoichiometry and thermochemistry. At A Level, an in-depth study of the electronic structure of atoms provides the basis for the study of chemical bonding. The Valence Shell Electron Pair Repulsion (VSEPR) model is used to visualis ...
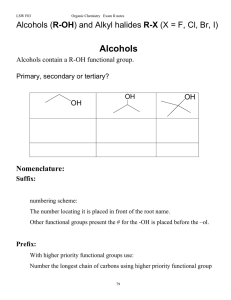
Alcohols (R-OH), and alkyl halides, RX
... How to calculate the ratio of products; Step 1: What are the possible monosubstituted products? Step 2: How many hydrogens that yield the same product? (nHi) Step 3: What is the reactivity (Ri) for the hydrogen and halogen type? Step 4: Using the reactivity factors, what are the total possible struc ...
... How to calculate the ratio of products; Step 1: What are the possible monosubstituted products? Step 2: How many hydrogens that yield the same product? (nHi) Step 3: What is the reactivity (Ri) for the hydrogen and halogen type? Step 4: Using the reactivity factors, what are the total possible struc ...
Microsoft Word
... identical conditions to produce 1,2,3-triazoles in good yields (Table 3). Encouraged by the results obtained with benzyl bromide, we turned our attention to various alkyl bromides. Interestingly, (2-bromoethyl)benzene and 1-bromopentane also participated well in this reaction. The effects of various ...
... identical conditions to produce 1,2,3-triazoles in good yields (Table 3). Encouraged by the results obtained with benzyl bromide, we turned our attention to various alkyl bromides. Interestingly, (2-bromoethyl)benzene and 1-bromopentane also participated well in this reaction. The effects of various ...
KINETICS questions
... (a) Complete the potential-energy diagram for reaction II on the graph above.. (b) For reaction I, predict how each of the following is affected as the temperature is increased by 20C. Explain the basis for each prediction. (i) Rate of reaction (ii) Heat of reaction (c) For reaction II, the form of ...
... (a) Complete the potential-energy diagram for reaction II on the graph above.. (b) For reaction I, predict how each of the following is affected as the temperature is increased by 20C. Explain the basis for each prediction. (i) Rate of reaction (ii) Heat of reaction (c) For reaction II, the form of ...
Organic Chemistry Lecture Outline Chapter 21: Carboxylic Acid
... 1. Anhydrides are usually prepared by reacting acyl chlorides with carboxylic acids in the presence of pyridine. C. Preparation of Esters: Esters can be prepared in three ways. 1. Fisher Esterification: Reaction of alcohols with a carboxylic acid in the presence of an acid catalyst. 2. Reaction of a ...
... 1. Anhydrides are usually prepared by reacting acyl chlorides with carboxylic acids in the presence of pyridine. C. Preparation of Esters: Esters can be prepared in three ways. 1. Fisher Esterification: Reaction of alcohols with a carboxylic acid in the presence of an acid catalyst. 2. Reaction of a ...
Chapter 4: Reaction Stoichiometry Reaction Stoichiometry
... Writing Reactions - Double Displacement Most precipitation and acid/base reactions are double displacement reactions. How do we write these reactions? 1. Split the reactants into individual ions. 2. Swap the cations to form new compounds these are your products. 3. Write the new compounds with bala ...
... Writing Reactions - Double Displacement Most precipitation and acid/base reactions are double displacement reactions. How do we write these reactions? 1. Split the reactants into individual ions. 2. Swap the cations to form new compounds these are your products. 3. Write the new compounds with bala ...
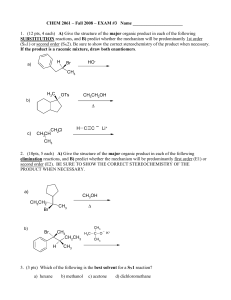
Exam #3
... (12 pts) Bromoetherification, the addition of the elements Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. Draw a stepwise mechanism for the following INTRAMOLECULAR bromoetherification reaction. Hint: the mechanism is analogous to that of bromohydrin f ...
... (12 pts) Bromoetherification, the addition of the elements Br and OR to a double bond, is a common method for constructing rings containing oxygen atoms. Draw a stepwise mechanism for the following INTRAMOLECULAR bromoetherification reaction. Hint: the mechanism is analogous to that of bromohydrin f ...
Copper-catalysed selective hydroamination reactions of alkynes Please share
... variety of strategies, including Sonogashira coupling13, nucleophilic addition of metal acetylides14, and homologation of carbonyl groups15. In addition, one or both π-bonds of alkynes may be utilized, further increasing their flexibility as starting materials. For these reasons, the hydrofunctional ...
... variety of strategies, including Sonogashira coupling13, nucleophilic addition of metal acetylides14, and homologation of carbonyl groups15. In addition, one or both π-bonds of alkynes may be utilized, further increasing their flexibility as starting materials. For these reasons, the hydrofunctional ...
SOL Review Part 3 Nomenclature reactions
... Step 3: Place remaining electrons on the outside atoms to fulfill octet rule ...
... Step 3: Place remaining electrons on the outside atoms to fulfill octet rule ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.