boehm_rl
... chlorine atoms were assumed to have substituted carbon-bound hydrogen atoms on carbon atoms one and four. The work of Barham, et al. did not indicate that carbon and hydrogen had been determined; these additional data might have given an indication of the oxygen remaining in the chlorostarches. ...
... chlorine atoms were assumed to have substituted carbon-bound hydrogen atoms on carbon atoms one and four. The work of Barham, et al. did not indicate that carbon and hydrogen had been determined; these additional data might have given an indication of the oxygen remaining in the chlorostarches. ...
Advanced Practical Organic Chemistry
... atoms can occur within organic molecules, these groups of atoms are called functional groups. One good example is the hydroxyl functional group. The hydroxyl group consists of a single oxygen atom bound to a single hydrogen atom (-OH). The group of hydrocarbons that contain a hydroxyl functional gro ...
... atoms can occur within organic molecules, these groups of atoms are called functional groups. One good example is the hydroxyl functional group. The hydroxyl group consists of a single oxygen atom bound to a single hydrogen atom (-OH). The group of hydrocarbons that contain a hydroxyl functional gro ...
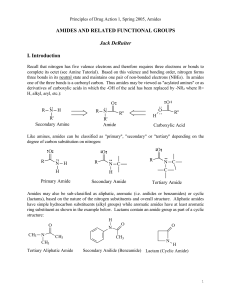
AMIDES AND RELATED FUNCTIONAL GROUPS
... hydrocarbons as illustrated below: While hydrogen bonding may enhance the water solubility of amides relative to hydrocarbons (alkanes, alkenes, alkynes and aromatic compounds), amides typically are regarded as compounds with low water solubility. They are significantly less water soluble than compa ...
... hydrocarbons as illustrated below: While hydrogen bonding may enhance the water solubility of amides relative to hydrocarbons (alkanes, alkenes, alkynes and aromatic compounds), amides typically are regarded as compounds with low water solubility. They are significantly less water soluble than compa ...
Ch. 10 Notes with Answers
... 6. View as carbanions: RMgBr = R Super Strong Bases and Nucleophiles • The counterion metal is a spectator • Stability-reactivity principle: very unstable ! very reactive • This great reactivity is very useful (as nucleophile) • This great reactivity (as base) has implication for proper technical us ...
... 6. View as carbanions: RMgBr = R Super Strong Bases and Nucleophiles • The counterion metal is a spectator • Stability-reactivity principle: very unstable ! very reactive • This great reactivity is very useful (as nucleophile) • This great reactivity (as base) has implication for proper technical us ...
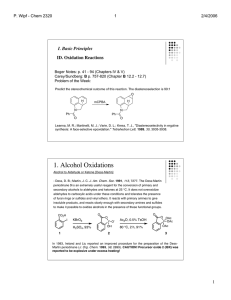
1. Alcohol Oxidations
... For a catalytic protocol, see: Bolm, C.; Magnus, A. S.; Hildebrand, J. P., "Catalytic synthesis of aldehydes and ketones under mild conditions using TEMPO/oxone." Org. Lett. 2000, 2, 1173-1175. TEMPO/NCS allows for the selective oxidation of primary over secondary alcohols: Einhorn, J.; Einhorn, C.; ...
... For a catalytic protocol, see: Bolm, C.; Magnus, A. S.; Hildebrand, J. P., "Catalytic synthesis of aldehydes and ketones under mild conditions using TEMPO/oxone." Org. Lett. 2000, 2, 1173-1175. TEMPO/NCS allows for the selective oxidation of primary over secondary alcohols: Einhorn, J.; Einhorn, C.; ...
PowerPoint 演示文稿
... and made many contributions to reaction mechanisms and molecular spectroscopy. Orientation and relative rates of aromatic nitration were used, in his early work, to test the theory. Studies of aliphatic substitutions and eliminations, often with his long-time collaborator E. D. Hughes, led to I ncor ...
... and made many contributions to reaction mechanisms and molecular spectroscopy. Orientation and relative rates of aromatic nitration were used, in his early work, to test the theory. Studies of aliphatic substitutions and eliminations, often with his long-time collaborator E. D. Hughes, led to I ncor ...
Nucleophilic Acyl Substitution
... Carboxylic acids containing six or fewer carbons are frequently called by their common names. These names were chosen by early chemists to describe some feature of the compound, usually its origin. For example, formic acid is found in ants, bees, and other stinging insects; its name comes from formi ...
... Carboxylic acids containing six or fewer carbons are frequently called by their common names. These names were chosen by early chemists to describe some feature of the compound, usually its origin. For example, formic acid is found in ants, bees, and other stinging insects; its name comes from formi ...
Synthesis of a TREN in Which the Aryl Substituents are... Atom Macrocycle ̈ller *
... ammonia) was found along with ∼1 equiv of hydrogen per N2 reduced. Eight of the proposed catalytic intermediates in the Mocatalyzed TREN-based system were characterized by X-ray crystallography. The proposed reaction mechanism also has been scrutinized through extensive calculations.2 All steric and ...
... ammonia) was found along with ∼1 equiv of hydrogen per N2 reduced. Eight of the proposed catalytic intermediates in the Mocatalyzed TREN-based system were characterized by X-ray crystallography. The proposed reaction mechanism also has been scrutinized through extensive calculations.2 All steric and ...
Transformation of Carbon Dioxide
... A widely accepted idea is that CO2 is so thermodynamically and kinetically stable that it is rarely used to its fullest potential. However, due to the electron deficiency of the carbonyl carbons, CO2 has a strong affinity toward nucleophiles and electron-donating reagents. In other words, CO2 is an ...
... A widely accepted idea is that CO2 is so thermodynamically and kinetically stable that it is rarely used to its fullest potential. However, due to the electron deficiency of the carbonyl carbons, CO2 has a strong affinity toward nucleophiles and electron-donating reagents. In other words, CO2 is an ...
Chemistry of alcohols (powerpoint)
... loss of a water molecule to generate a carbocation (carbonium ion) a bromide ion behaves as a nucleophile and attacks the carbocation ...
... loss of a water molecule to generate a carbocation (carbonium ion) a bromide ion behaves as a nucleophile and attacks the carbocation ...
Efficient hydrogenation of organic carbonates, carbamates and
... groups, has captured much attention during the past four decades1–3, mainly due to its synthetic significance as an environmentally benign approach to fundamental synthetic building blocks such as alcohols and amines. Much progress has been made in the hydrogenation of ketones and aldehydes and, more ...
... groups, has captured much attention during the past four decades1–3, mainly due to its synthetic significance as an environmentally benign approach to fundamental synthetic building blocks such as alcohols and amines. Much progress has been made in the hydrogenation of ketones and aldehydes and, more ...
organic practice problems
... c. CF2CH2 d. CF2CF2 e. CHFCF2 ____ 79. All of the following statements concerning polymers are correct EXCEPT a. elastomers are materials that spring back to their original shape when stretched. b. polymers formed from two or more different monomers are called copolymers. c. thermoplastics can withs ...
... c. CF2CH2 d. CF2CF2 e. CHFCF2 ____ 79. All of the following statements concerning polymers are correct EXCEPT a. elastomers are materials that spring back to their original shape when stretched. b. polymers formed from two or more different monomers are called copolymers. c. thermoplastics can withs ...
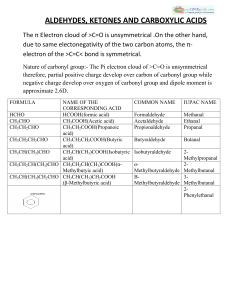
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
... :-Aldehydes, Ketones and Carboxylic acids are important classes of organic compounds containing carbonyl groups. :-They are highly polar molecules. :-They boil at higher temperatures than the corresponding hydrocarbons and weakly polar compounds such as ethers. :-Lower members are soluble in water b ...
... :-Aldehydes, Ketones and Carboxylic acids are important classes of organic compounds containing carbonyl groups. :-They are highly polar molecules. :-They boil at higher temperatures than the corresponding hydrocarbons and weakly polar compounds such as ethers. :-Lower members are soluble in water b ...
1 Q. What are Saturated and unsaturated hydrocarbons?imp
... Staggered conformations has the lease torsional strain while eclipsed having maximum. Due to torsional strain, certain energy called torsional energy, is required to allow the rotation around the C-C single bond. in other words ethane molecule having staggered conformation will have to come cross an ...
... Staggered conformations has the lease torsional strain while eclipsed having maximum. Due to torsional strain, certain energy called torsional energy, is required to allow the rotation around the C-C single bond. in other words ethane molecule having staggered conformation will have to come cross an ...
SYNTHESIS OF NEW DICLOFENAC DERIVATIVES BY COUPLING WITH CHALCONE
... are based in general on the formation of carbon-carbon bond and here it is the Enone moiety (i.e., the α,β-unsaturated ketone). Among other strategies, the Claisen-Schmidt condensation appeared to be the most appealing one, where it involves the condensation of an aromatic ketone with an aromatic al ...
... are based in general on the formation of carbon-carbon bond and here it is the Enone moiety (i.e., the α,β-unsaturated ketone). Among other strategies, the Claisen-Schmidt condensation appeared to be the most appealing one, where it involves the condensation of an aromatic ketone with an aromatic al ...
Efficient and Convenient Procedure for Protection of Hydroxyl
... high sterically hindrance, remained unaffected even when the reaction mixtures were stirred at room temperature for one day, and the starting materials were quantitatively recovered. The reaction conditions are mild enough not to induce any isomerization for conjugated alcohols or damage to moieties ...
... high sterically hindrance, remained unaffected even when the reaction mixtures were stirred at room temperature for one day, and the starting materials were quantitatively recovered. The reaction conditions are mild enough not to induce any isomerization for conjugated alcohols or damage to moieties ...
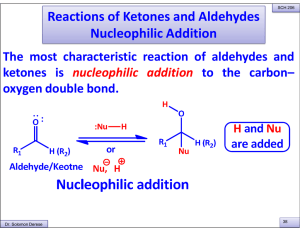
Reactions of Ketones and Aldehydes Nucleophilic Addition
... If the cassava is crushed with water and allowed to stand (‘ferment’), enzymes in the cassava will do the same job and then the HCN can be washed out before the cassava is cooked and eaten. The cassava is now safe to eat but it still contains some glucoside. Some diseases found in eastern Nigeria ca ...
... If the cassava is crushed with water and allowed to stand (‘ferment’), enzymes in the cassava will do the same job and then the HCN can be washed out before the cassava is cooked and eaten. The cassava is now safe to eat but it still contains some glucoside. Some diseases found in eastern Nigeria ca ...
Alcohols, Phenols, and Thiols
... Alcohols are comparable in acidity to water, but phenols are much more acidic. This increased acidity is due to charge delocalization (resonance) in phenoxide ions. Electron-withdrawing groups, such as F and NO2, increase acidity, through either an inductive or a resonance effect, or both. Alkoxid ...
... Alcohols are comparable in acidity to water, but phenols are much more acidic. This increased acidity is due to charge delocalization (resonance) in phenoxide ions. Electron-withdrawing groups, such as F and NO2, increase acidity, through either an inductive or a resonance effect, or both. Alkoxid ...
Problem 1-2
... gas C burns with a light blue flame. The elementary analysis of B shows 24.5 % (w/w) of carbon and 28.6 % (w/w) of nitrogen. When annealed with carbon another ionic compound D also results in compound B, too, but without the gas. D reacts with acids to form urea among other substances. The elementar ...
... gas C burns with a light blue flame. The elementary analysis of B shows 24.5 % (w/w) of carbon and 28.6 % (w/w) of nitrogen. When annealed with carbon another ionic compound D also results in compound B, too, but without the gas. D reacts with acids to form urea among other substances. The elementar ...
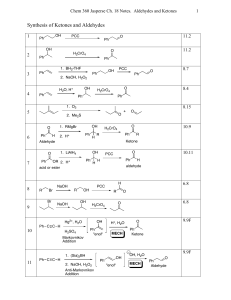
Synthesis of Ketones and Aldehydes
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
... Classification of Mechanisms Associated With Ketone/Aldehyde Reactions. • There may seem to be a dizzying number of mechanisms this chapter. But all of them simplify into some combination of acid- or base-catalyzed addition reaction, elimination reaction and/or substitution reaction. • To predict wh ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.