Making Scents of Esters
... Making Scents of Esters Introduction: An ester is an organic compound that is formed, in addition to water, when a carboxylic acid reacts with an alcohol. This process is called esterification. General Reaction: ...
... Making Scents of Esters Introduction: An ester is an organic compound that is formed, in addition to water, when a carboxylic acid reacts with an alcohol. This process is called esterification. General Reaction: ...
This is an author version of the contribution published on: Questa è
... Preliminary efforts were directed towards the formation of neryl, geranyl and farnesyl acetate, starting from the respective alcohols and acetic acid using 1-ethyl-3methylimidazolium methanesulfonate (EMImMeSO3) as both solvent and acidic catalyst (Scheme 1). At first, the reactions were performed u ...
... Preliminary efforts were directed towards the formation of neryl, geranyl and farnesyl acetate, starting from the respective alcohols and acetic acid using 1-ethyl-3methylimidazolium methanesulfonate (EMImMeSO3) as both solvent and acidic catalyst (Scheme 1). At first, the reactions were performed u ...
Carboxylic Acids - BSAK Chemistry weebly
... • Benzoyl chloride has the formula C6H5COCl. • How does the reactivity of benzoyl chloride compare to that of ethanoyl chloride? Explain. • The -COCl group is attached directly to a benzene ring. It is much less reactive than simple acyl chlorides like ethanoyl chloride. ...
... • Benzoyl chloride has the formula C6H5COCl. • How does the reactivity of benzoyl chloride compare to that of ethanoyl chloride? Explain. • The -COCl group is attached directly to a benzene ring. It is much less reactive than simple acyl chlorides like ethanoyl chloride. ...
- Cypress HS
... Many chemical reactions, especially those of organic substances, do not go to completion. Rather, they come to a point of chemical equilibrium before the reactants are fully converted to products. At the point of equilibrium, the concentrations of all reactants remain constant with time. The positio ...
... Many chemical reactions, especially those of organic substances, do not go to completion. Rather, they come to a point of chemical equilibrium before the reactants are fully converted to products. At the point of equilibrium, the concentrations of all reactants remain constant with time. The positio ...
Functional Groups
... Rule #1: Identify the longest chain of carbon atoms a) The longest chain of carbon atoms gives the stem/root of the name as shown in the table below: # of C-atoms in Stem in longest chain IUPAC name ...
... Rule #1: Identify the longest chain of carbon atoms a) The longest chain of carbon atoms gives the stem/root of the name as shown in the table below: # of C-atoms in Stem in longest chain IUPAC name ...
Topic checklist
... The relative amounts of energy released when substances burn can be measured by simple calorimetry, eg by heating water in a glass or metal container. This method can be used to compare the amount of energy produced by fuels and foods. Energy is normally measured in joules (J). The amount of energy ...
... The relative amounts of energy released when substances burn can be measured by simple calorimetry, eg by heating water in a glass or metal container. This method can be used to compare the amount of energy produced by fuels and foods. Energy is normally measured in joules (J). The amount of energy ...
27_04_06.html
... Decarboxylation is a common reaction of aamino acids. An example is the conversion of L-histidine to histamine. Antihistamines act by blocking the action of histamine. ...
... Decarboxylation is a common reaction of aamino acids. An example is the conversion of L-histidine to histamine. Antihistamines act by blocking the action of histamine. ...
4. Amines Amides and Amino Acids
... substituted onto the N atom in place of H atoms. Therefore more electron density is pushed onto the N atom (as the inductive effect of alkyl groups is greater than that of H atoms). One might expect using the same trend that tertiary amine would be the strongest amine base but the trend does not hol ...
... substituted onto the N atom in place of H atoms. Therefore more electron density is pushed onto the N atom (as the inductive effect of alkyl groups is greater than that of H atoms). One might expect using the same trend that tertiary amine would be the strongest amine base but the trend does not hol ...
CBS Reduction
... Recent paper 2016 • Sanderson and coworkers developed flow process for CBS asymmetric reduction of ketones , using chip-microreactors. • They used BH3 , (85% 2-MeTHF, 15% THF) and oxazaborolidine for reduction. • Under such reaction conditions, the reaction was complete in 10 minutes and alcohol wa ...
... Recent paper 2016 • Sanderson and coworkers developed flow process for CBS asymmetric reduction of ketones , using chip-microreactors. • They used BH3 , (85% 2-MeTHF, 15% THF) and oxazaborolidine for reduction. • Under such reaction conditions, the reaction was complete in 10 minutes and alcohol wa ...
Chapter 3 Carboxylic Acids
... stronger in carboxylic acids than in other compounds of similar shape and molecular weight. ...
... stronger in carboxylic acids than in other compounds of similar shape and molecular weight. ...
aldehydes and ketones
... The reactivity of carbonyl compounds is also related to the basicity of Y–: (lone pair of an atom) ...
... The reactivity of carbonyl compounds is also related to the basicity of Y–: (lone pair of an atom) ...
Organic Chemistry
... Organic Compounds and the Atomic Properties of Carbon 15.1 The Special Nature of Carbon and the Characteristics of Organic Molecules 15.2 The Structures and Classes of Hydrocarbons 15.3 Some Important Classes of Organic Reactions 15.4 Properties and Reactivities of Common Functional Groups 15.5 The ...
... Organic Compounds and the Atomic Properties of Carbon 15.1 The Special Nature of Carbon and the Characteristics of Organic Molecules 15.2 The Structures and Classes of Hydrocarbons 15.3 Some Important Classes of Organic Reactions 15.4 Properties and Reactivities of Common Functional Groups 15.5 The ...
ch15
... Organic Compounds and the Atomic Properties of Carbon 15.1 The Special Nature of Carbon and the Characteristics of Organic Molecules 15.2 The Structures and Classes of Hydrocarbons 15.3 Some Important Classes of Organic Reactions 15.4 Properties and Reactivities of Common Functional Groups 15.5 The ...
... Organic Compounds and the Atomic Properties of Carbon 15.1 The Special Nature of Carbon and the Characteristics of Organic Molecules 15.2 The Structures and Classes of Hydrocarbons 15.3 Some Important Classes of Organic Reactions 15.4 Properties and Reactivities of Common Functional Groups 15.5 The ...
EXPERIMENT 4 Objectives Principles
... of electrons, or ions with a negative charge. They can form bond by donating electrons to another molecule having a position of lower electron density (electrophiles). Examples of nucleophilic species are: water, amine, ammonia, cyanide ion, alkoxide ion, and hydroxide ion. Alkyl halides can react w ...
... of electrons, or ions with a negative charge. They can form bond by donating electrons to another molecule having a position of lower electron density (electrophiles). Examples of nucleophilic species are: water, amine, ammonia, cyanide ion, alkoxide ion, and hydroxide ion. Alkyl halides can react w ...
+ CH - Loreto Chemistry from 2015
... (a) explanation of the water solubility of carboxylic acids in terms of hydrogen bonding (b) reactions in aqueous conditions of carboxylic acids with metals and bases (including carbonates, metal oxides and alkalis) (c) esterification of: (i) carboxylic acids with alcohols in the presence of an acid ...
... (a) explanation of the water solubility of carboxylic acids in terms of hydrogen bonding (b) reactions in aqueous conditions of carboxylic acids with metals and bases (including carbonates, metal oxides and alkalis) (c) esterification of: (i) carboxylic acids with alcohols in the presence of an acid ...
Chapter 21 The Chemistry of Carboxylic Acid Derivatives
... The reaction of a Grignard reagent with ethyl formate gives a secondary alcohol in which the two alkyl groups at the a-carbon are identical. ...
... The reaction of a Grignard reagent with ethyl formate gives a secondary alcohol in which the two alkyl groups at the a-carbon are identical. ...
Synthesis of Amino Acid Methyl Ester Hydrochloride
... the air. The methanol is cooled in an ice-bath for 1-2 min. Thionyl chloride, handle with care, see above (0.52 mL) is drawn up into a 1 mL graduated syringe with polyethylene tube tip, as described in separate procedure B (appendix), and is cautiously added to the methanol over a period of approxim ...
... the air. The methanol is cooled in an ice-bath for 1-2 min. Thionyl chloride, handle with care, see above (0.52 mL) is drawn up into a 1 mL graduated syringe with polyethylene tube tip, as described in separate procedure B (appendix), and is cautiously added to the methanol over a period of approxim ...
File
... Elimination reaction: start with one organic molecule and end up with a product that contains a double or triple bond and a small molecule o The opposite of an addition reaction o We only looked at elimination of alcohols and alkyl halides o Know the required conditions for each Substitution reactio ...
... Elimination reaction: start with one organic molecule and end up with a product that contains a double or triple bond and a small molecule o The opposite of an addition reaction o We only looked at elimination of alcohols and alkyl halides o Know the required conditions for each Substitution reactio ...
Elimination Reactions
... reactions are useful because alkenes are used to manufacture many products such as: Carpets Clothing ...
... reactions are useful because alkenes are used to manufacture many products such as: Carpets Clothing ...
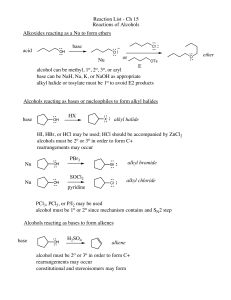
Reaction List - Ch 15 Reactions of Alcohols Alkoxides reacting as a
... alcohol can be methyl, 1o, 2o, 3o, or aryl base can be NaH, Na, K, or NaOH as appropriate alkyl halide or tosylate must be 1o to avoid E2 products Alcohols reacting as bases or nucleophiles to form alkyl halides base ...
... alcohol can be methyl, 1o, 2o, 3o, or aryl base can be NaH, Na, K, or NaOH as appropriate alkyl halide or tosylate must be 1o to avoid E2 products Alcohols reacting as bases or nucleophiles to form alkyl halides base ...
Ethers and Epoxides
... • A large portion of the materials on plagiarism on the University of Wisconsin Oshkosh's Writing Center Web site was revealed in February to have been taken verbatim from Purdue University's Web page on plagiarism. ...
... • A large portion of the materials on plagiarism on the University of Wisconsin Oshkosh's Writing Center Web site was revealed in February to have been taken verbatim from Purdue University's Web page on plagiarism. ...
Abbreviated Chapter 17 Powerpoint
... • If the substituent on the ring is electron donating, the ortho and para positions will be activated. • If the group is electron withdrawing, the ortho and para positions will be deactivated. ...
... • If the substituent on the ring is electron donating, the ortho and para positions will be activated. • If the group is electron withdrawing, the ortho and para positions will be deactivated. ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.