Exam 3 - Organic Chemistry at CU Boulder
... stereochemistry if appropriate. If a racemate is formed, show only one enantiomer, and label it “rac”. Assume chiral starting materials are single pure enantiomers (3 pts each) ...
... stereochemistry if appropriate. If a racemate is formed, show only one enantiomer, and label it “rac”. Assume chiral starting materials are single pure enantiomers (3 pts each) ...
Student Instructions from Laboratory Manual
... If you wish to further purify your product by crystallization, a good place to start is by chilling a concentrated solution of the product in diethyl ether or pentane. ...
... If you wish to further purify your product by crystallization, a good place to start is by chilling a concentrated solution of the product in diethyl ether or pentane. ...
Kazzie`s Guide to Orgo 2
... General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 210 Stuff Identify ...
... General Note: Some of these questions have been previously used in examples, etcetera, but they cover the things that I think are important to know from this semester. Try to work through them with as few resources as possible, and we will go through this at the final review. Chem 210 Stuff Identify ...
ASYMMETRIC CATALYSIS
... a platform for unexpected discoveries if a flexible plan can be executed. Although some effective ligands have been developed, no universal chiral ligand or catalyst exists for solving all problems in enantioselective transformations. Strategically important asymmetric catalytic reactions can be dev ...
... a platform for unexpected discoveries if a flexible plan can be executed. Although some effective ligands have been developed, no universal chiral ligand or catalyst exists for solving all problems in enantioselective transformations. Strategically important asymmetric catalytic reactions can be dev ...
Chapter 22/23-Organic Chemistry
... c. 1,3-dibromo-2-chloro-5-methylcylcohexane d. 3,3-difluoro-1,4-pentadiene e. 4–ethyl–2,6–dimethyl–2–heptene. f. 1,1-dichlorobenzene 3. Based on what you know about the ability of a carbon-carbon double bond to rotate, do these two structures represent isomers, or are they just the same molecule? ...
... c. 1,3-dibromo-2-chloro-5-methylcylcohexane d. 3,3-difluoro-1,4-pentadiene e. 4–ethyl–2,6–dimethyl–2–heptene. f. 1,1-dichlorobenzene 3. Based on what you know about the ability of a carbon-carbon double bond to rotate, do these two structures represent isomers, or are they just the same molecule? ...
Chemistry Crunch #12.2: Organic Reactions KEY Why? Learning
... 3. Summarize. In a characteristic addition reaction: a) There will always be 2 reactant(s) and 1 product(s). b) The hydrocarbon reactant will always have a double or triple bond, or in other words, the hydrocarbon will be unsaturated. c) We learned how to classify many non-organic chemical reactions ...
... 3. Summarize. In a characteristic addition reaction: a) There will always be 2 reactant(s) and 1 product(s). b) The hydrocarbon reactant will always have a double or triple bond, or in other words, the hydrocarbon will be unsaturated. c) We learned how to classify many non-organic chemical reactions ...
Lecture 9a - University of California, Los Angeles
... reactive than ketones which means that both groups would react with the Grignard reagent, albeit with different rates The higher reactivity of the aldehyde is exploited in the formation of the cyclic acetal using 1,3-propanediol ...
... reactive than ketones which means that both groups would react with the Grignard reagent, albeit with different rates The higher reactivity of the aldehyde is exploited in the formation of the cyclic acetal using 1,3-propanediol ...
Materials Seminar Professor Carsten Sievers Georgia Institute of Technology
... (HDO) of bio-oils and the direct conversion of methane to higher alcohols. HDO is a promising route for converting complex mixtures of oxygenates in bio-oils to biofuels. The process provides oils with reduced reactivity and corrosiveness and increases the energy density of the product. In HDO, oxyg ...
... (HDO) of bio-oils and the direct conversion of methane to higher alcohols. HDO is a promising route for converting complex mixtures of oxygenates in bio-oils to biofuels. The process provides oils with reduced reactivity and corrosiveness and increases the energy density of the product. In HDO, oxyg ...
Here is the Original File - University of New Hampshire
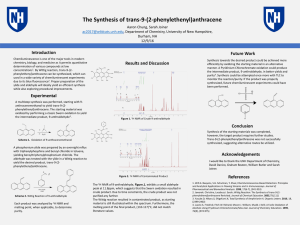
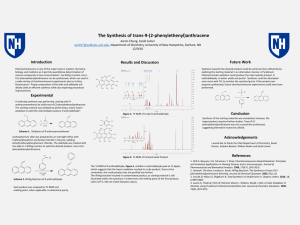
... chemistry, biology, and medicine as it permits quantitative determination of various compounds at low concentrations1. By Wittig reaction, trans-9-(2phenylethenyl)anthracene can be synthesized, which can used in a wide variety of chemiluminescent experiments due to its blue fluorescence2. Proper pre ...
... chemistry, biology, and medicine as it permits quantitative determination of various compounds at low concentrations1. By Wittig reaction, trans-9-(2phenylethenyl)anthracene can be synthesized, which can used in a wide variety of chemiluminescent experiments due to its blue fluorescence2. Proper pre ...
The Synthesis of trans-9-(2
... Chemiluminescence is one of the major tools in modern chemistry, biology, and medicine as it permits quantitative determination of various compounds at low concentrations1. By Wittig reaction, trans9-(2-phenylethenyl)anthracene can be synthesized, which can used in a wide variety of chemiluminescent ...
... Chemiluminescence is one of the major tools in modern chemistry, biology, and medicine as it permits quantitative determination of various compounds at low concentrations1. By Wittig reaction, trans9-(2-phenylethenyl)anthracene can be synthesized, which can used in a wide variety of chemiluminescent ...
- M E S KVM College Valanchery.
... (a) CH3CH2ONa : No, since it is an oxygen-metal bond (b) CH3CH2Li : Yes there is a carbon-metal bond (c) CH3CH2BH2 : No, boron is a non-metal (d) (CH3CH2)2Zn : Yes there are 2 carbon-metal bonds (e) CH3CH2MgBr : Yes there is a carbon-metal bond (f) CH3C≡CNa : Yes there is a carbon-metal bond ...
... (a) CH3CH2ONa : No, since it is an oxygen-metal bond (b) CH3CH2Li : Yes there is a carbon-metal bond (c) CH3CH2BH2 : No, boron is a non-metal (d) (CH3CH2)2Zn : Yes there are 2 carbon-metal bonds (e) CH3CH2MgBr : Yes there is a carbon-metal bond (f) CH3C≡CNa : Yes there is a carbon-metal bond ...
Survey on Conditions Catalysis of Chemical Reactions
... will reduce aldehydes, ketones, esters, carboxylic acid chlorides, carboxylic acids and even carboxylate salts to alcohols. Amides and nitriles are reduced to amines. In each case the partially negative hydrogen reacts with the partially positive carbon of the substrate. It can also be used to reduc ...
... will reduce aldehydes, ketones, esters, carboxylic acid chlorides, carboxylic acids and even carboxylate salts to alcohols. Amides and nitriles are reduced to amines. In each case the partially negative hydrogen reacts with the partially positive carbon of the substrate. It can also be used to reduc ...
Exp 19 - Diphenylacetylene_2015
... dibromide. What is the percent yield of the reaction? See supplemental handout for help. 3. How does the addition of cyclohexene destroy excess bromine in the solution? 4. In the second procedure, you are instructed to add enough triethylene glycol (TEG) to make the solution 0.6 M in meso-stilbene d ...
... dibromide. What is the percent yield of the reaction? See supplemental handout for help. 3. How does the addition of cyclohexene destroy excess bromine in the solution? 4. In the second procedure, you are instructed to add enough triethylene glycol (TEG) to make the solution 0.6 M in meso-stilbene d ...
Organic Reactions Worksheet
... iv) Write the balanced equation for each oxidizing reaction, use [O] convention c. Using any secondary alcohol: i) Give the displayed (structural formula) which it could be oxidized to ii) State which homologous series the products are part of iii) Write the balanced equation for each oxidizing reac ...
... iv) Write the balanced equation for each oxidizing reaction, use [O] convention c. Using any secondary alcohol: i) Give the displayed (structural formula) which it could be oxidized to ii) State which homologous series the products are part of iii) Write the balanced equation for each oxidizing reac ...
Name__________________________Review Organic Reactions
... 14. Which organic compounds are often used to create fragrances for the perfume industry? A) ethers C) alkanes ...
... 14. Which organic compounds are often used to create fragrances for the perfume industry? A) ethers C) alkanes ...
chemistry 2 - waiukucollegescience
... In order to distinguish between propan-1-ol and propene a student said it was necessary to use bromine water rather than acidified potassium permanganate. Discuss this statement. ...
... In order to distinguish between propan-1-ol and propene a student said it was necessary to use bromine water rather than acidified potassium permanganate. Discuss this statement. ...
Chapter 7
... atoms in the T.S. of an E2 reaction must lie in the same plane. • There are two ways this can happen: ...
... atoms in the T.S. of an E2 reaction must lie in the same plane. • There are two ways this can happen: ...
Chapter 21: Carboxylic Acid Derivatives and Nucleophilic Acyl
... Relative reactivity – substituent effects ...
... Relative reactivity – substituent effects ...
Semester II
... structure elucidation, important reaction of metal carbonyls, Metal nitrosyls: Nitrosylating agents for synthesis of metal nitrosyls, vibrational spectra and X-ray diffraction studies of transition metal nitrosyls for bonding and structure elucidation, important reactions of transition metal nitrosy ...
... structure elucidation, important reaction of metal carbonyls, Metal nitrosyls: Nitrosylating agents for synthesis of metal nitrosyls, vibrational spectra and X-ray diffraction studies of transition metal nitrosyls for bonding and structure elucidation, important reactions of transition metal nitrosy ...
Types of Chemical Reactions
... (solid, liquid, aqueous, or gas). If no reaction occurs write the words "no reaction" (or NR) instead of the products in your balanced equation and indicate why your think there was no reaction. Unless otherwise indicated, dispose of all waste in the waste container, or a beaker that you pour into t ...
... (solid, liquid, aqueous, or gas). If no reaction occurs write the words "no reaction" (or NR) instead of the products in your balanced equation and indicate why your think there was no reaction. Unless otherwise indicated, dispose of all waste in the waste container, or a beaker that you pour into t ...
MULTISTEP SYNTHESIS PROTECTING GROUPS
... Sulfonyl chlorides contain a good leaving group (Cl). That is what makes them highly reactive towards water and other nucleophiles such as ammonia (NH3). These reactions are used in this synthesis, but they can also cause problems. For example, the second step of the synthesis transforms p-acetamido ...
... Sulfonyl chlorides contain a good leaving group (Cl). That is what makes them highly reactive towards water and other nucleophiles such as ammonia (NH3). These reactions are used in this synthesis, but they can also cause problems. For example, the second step of the synthesis transforms p-acetamido ...
R-c-H+H-oH:n-J-u oo o il o o o I o
... 7. One mole of methanolwould releasemore energy upon complete oxidation than one mole of methane. B. Both aldehydesand ketones have a carbonyl group. 9. All aldehydesand ketones give a positive Tollens' test. 10. Fourpairs ofelectrons are sharedinthe carbonyl bond formed between an oxygen and a carb ...
... 7. One mole of methanolwould releasemore energy upon complete oxidation than one mole of methane. B. Both aldehydesand ketones have a carbonyl group. 9. All aldehydesand ketones give a positive Tollens' test. 10. Fourpairs ofelectrons are sharedinthe carbonyl bond formed between an oxygen and a carb ...
organometallic reagents
... product is to work the synthesis backwards on paper. This approach is called retrosynthetic analysis. In this approach, strategic C-C bonds in the target molecule are broken at points where bond formation seems possible. The reason that retrosynthetic analysis is useful is that fewer possible reacti ...
... product is to work the synthesis backwards on paper. This approach is called retrosynthetic analysis. In this approach, strategic C-C bonds in the target molecule are broken at points where bond formation seems possible. The reason that retrosynthetic analysis is useful is that fewer possible reacti ...
Chemistry (Theory)
... When aldol condensation is carried out between two different aldehydes and / or ketones, it is called cross aldol condensation. If both of them contain α-hydrogen atoms, it gives a mixture of four products. ...
... When aldol condensation is carried out between two different aldehydes and / or ketones, it is called cross aldol condensation. If both of them contain α-hydrogen atoms, it gives a mixture of four products. ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.