Fluorinated Butatrienes - diss.fu-berlin.de
... wurde ein Enin-Isomer entdeckt, das erstaunlicherweise stabiler als sein Butatrien Isomer ist, obwohl es an der Dreifachbindung fluoriert ist. Eben jene Fluorierung an der Dreifachbindung ist eigentlich notwendig um die Energie fluorierter But-1-en-3-ine relativ ...
... wurde ein Enin-Isomer entdeckt, das erstaunlicherweise stabiler als sein Butatrien Isomer ist, obwohl es an der Dreifachbindung fluoriert ist. Eben jene Fluorierung an der Dreifachbindung ist eigentlich notwendig um die Energie fluorierter But-1-en-3-ine relativ ...
Liquid-gas phase-boundary catalytic system
... simulation theory. The use of TiO2 as inorganic precursor and organic surfactant, however, has not been reported. In our recent report [5 ], well-aligned titanium dioxide was successfully synthesized by sol-gel method by using tetra-n-butyl orthotitanate (TBOT) as titanium dioxide precursor. Wellali ...
... simulation theory. The use of TiO2 as inorganic precursor and organic surfactant, however, has not been reported. In our recent report [5 ], well-aligned titanium dioxide was successfully synthesized by sol-gel method by using tetra-n-butyl orthotitanate (TBOT) as titanium dioxide precursor. Wellali ...
Carboxylic acids from primary alcohols and aldehydes by a
... quantitative yields. Benzylic (entries 1, 4–7 and 10), aliphatic (entries 2 and 12) as well as homobenzylic (entries 3, 8, 9, and 11) alcohols oxidized smoothly in a short amount of time. Electron-poor (entries 5 and 6) as well as electron-rich (entry 4 and 7) benzyl alcohols were oxidized without a ...
... quantitative yields. Benzylic (entries 1, 4–7 and 10), aliphatic (entries 2 and 12) as well as homobenzylic (entries 3, 8, 9, and 11) alcohols oxidized smoothly in a short amount of time. Electron-poor (entries 5 and 6) as well as electron-rich (entry 4 and 7) benzyl alcohols were oxidized without a ...
Microsoft Word
... NMR, GPC, TG/DTA, and DSC. # Kinetics, synthesis and characterization of amphiphilic poly(ethylene oxide) block copolymers by atom transfer radical polymerization. The macroinitiators and their block copolymers were characterized by FT-IR, 1 H & 13 C NMR, MALDI TOF-MS, GPC, TG/DTA, DSC and SEM. PART ...
... NMR, GPC, TG/DTA, and DSC. # Kinetics, synthesis and characterization of amphiphilic poly(ethylene oxide) block copolymers by atom transfer radical polymerization. The macroinitiators and their block copolymers were characterized by FT-IR, 1 H & 13 C NMR, MALDI TOF-MS, GPC, TG/DTA, DSC and SEM. PART ...
Chapter 18
... Depending upon the conditions used to protonate the alkoxide in the previous step, ...
... Depending upon the conditions used to protonate the alkoxide in the previous step, ...
... Aminoacylase I (Acy-I, EC 3.5.1.14) is found in many mammalian tissues, with the highest activity occurring in kidney [5]. The enzyme hydrolyzes a variety of N-acylated amino acids; however, the physiological role and the exact cellular localization of Acy-I is still a matter of debate. The comparis ...
Carbonyl The carbonyl function, C=O, exists in a number of organic
... involved are somewhat cloudy. As shown below the reagents add in a fast step to form an eclipsed betaine (or goes directly to the oxaphosphetane). The oxaphosphetane breaks down by syn elimination to give the trans (E) product. In a slower step the staggered more stable betaine is formed that leads ...
... involved are somewhat cloudy. As shown below the reagents add in a fast step to form an eclipsed betaine (or goes directly to the oxaphosphetane). The oxaphosphetane breaks down by syn elimination to give the trans (E) product. In a slower step the staggered more stable betaine is formed that leads ...
CC 2 097-110..7686hdisk chapter .. Page97
... groups on the benzene ring on electronic excitation (for the photodecarboxylation reactions reported), although it was not clear whether this characteristic is best attributed to its S1 or T1 state or both.† Although ketones classically react via their triplet excited states, typically via Type I an ...
... groups on the benzene ring on electronic excitation (for the photodecarboxylation reactions reported), although it was not clear whether this characteristic is best attributed to its S1 or T1 state or both.† Although ketones classically react via their triplet excited states, typically via Type I an ...
Chapter 9 Stoichiometry
... 5. Calculate the mass of a reactant or product from the mass of a different reactant or product. 6. Describe a method for determining which of two reactants is a limiting reactant. 7. Calculate the amount in moles of a product produced, given the amounts in moles of two reactants, one of which is in ...
... 5. Calculate the mass of a reactant or product from the mass of a different reactant or product. 6. Describe a method for determining which of two reactants is a limiting reactant. 7. Calculate the amount in moles of a product produced, given the amounts in moles of two reactants, one of which is in ...
Organic Chemistry II / CHEM 252 Chapter 16
... – The tetrahedral carbon resulting from addition to an aldehyde is less sterically hindered than the tetrahedral carbon resulting from addition to a ketone – Aldehyde carbonyl groups are more electron deficient because they have only one electron-donating group attached to the carbonyl carbon ...
... – The tetrahedral carbon resulting from addition to an aldehyde is less sterically hindered than the tetrahedral carbon resulting from addition to a ketone – Aldehyde carbonyl groups are more electron deficient because they have only one electron-donating group attached to the carbonyl carbon ...
Alkene-Addn-PartB-2012-ques
... The correlary is that an optically active starting material MAY produce an optically active product depending on the mechanism. ...
... The correlary is that an optically active starting material MAY produce an optically active product depending on the mechanism. ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition
... • A tetrahedral alkoxide ion intermediate is produced ...
... • A tetrahedral alkoxide ion intermediate is produced ...
Chapter 19 - people.vcu.edu
... Pyrrole is even less basic, with a pKb around 16, because its lone pair is involved in aromaticity. o EDGs will increase basicity, while EWGs will decrease basicity. o My weird way of thinking about basicity: The lone pair of a base is like teeth that want to bite a proton. The bigger the teet ...
... Pyrrole is even less basic, with a pKb around 16, because its lone pair is involved in aromaticity. o EDGs will increase basicity, while EWGs will decrease basicity. o My weird way of thinking about basicity: The lone pair of a base is like teeth that want to bite a proton. The bigger the teet ...
Nucleophilic Substitution and b
... Expect mechanism to be protonation of alkene to yield more stable carbocation followed by reaction with the weakly nucleophilic alcohol. ...
... Expect mechanism to be protonation of alkene to yield more stable carbocation followed by reaction with the weakly nucleophilic alcohol. ...
Chapter 22 Alpha Substitution and Condensations of Enols
... • When C=C is conjugated with C=O, 1,2-addition or 1,4-addition may occur. • A 1,4-addition of an enolate ion is called the Michael reaction. ...
... • When C=C is conjugated with C=O, 1,2-addition or 1,4-addition may occur. • A 1,4-addition of an enolate ion is called the Michael reaction. ...
Lecture 3-edited
... Chromium is the 21st most abundant element in Earth's crust with atomic number 24. Naturally occurring chromium composed of three stable isotopes; 52Cr, 53Cr and 54Cr with 52Cr being most abundant. It has an electronic configuration of 3d5 4s1 and exhibits a wide range of oxidation states, where the ...
... Chromium is the 21st most abundant element in Earth's crust with atomic number 24. Naturally occurring chromium composed of three stable isotopes; 52Cr, 53Cr and 54Cr with 52Cr being most abundant. It has an electronic configuration of 3d5 4s1 and exhibits a wide range of oxidation states, where the ...
PowerPoint Presentation - Chapter 1
... available to (CH3)3CBr are SN1 and E1 B) the mechanism generally believed to be available to (CH3)3CBr are SN1, SN2 and E1 C) the mechanism generally believed to be available to (CH3)3CBr are SN1, SN2 and E2 D) the mechanism generally believed to be available to (CH3)3CBr are SN1, E1 and E2 ...
... available to (CH3)3CBr are SN1 and E1 B) the mechanism generally believed to be available to (CH3)3CBr are SN1, SN2 and E1 C) the mechanism generally believed to be available to (CH3)3CBr are SN1, SN2 and E2 D) the mechanism generally believed to be available to (CH3)3CBr are SN1, E1 and E2 ...
... NMR spectroscopes as well as conductometric titrations and thermal analyses. To determine the effect of the nature of the coordinated metal atoms in the coordination sphere of L upon sulfur nucleophilicity and the rate of thiolate methylation. We describe herein the synthesis of a series of LM(II) t ...
Boron Reagents in Process Chemistry: Excellent
... hydroborated a terminal olefin with dicylcohexylborane followed by oxidation to give gram quantities of the alcohol 27, a key intermediate in the synthesis of epothilone A (Scheme 11).26 Notice that the internal alkenyl group in 26 was not hydroborated under the reaction conditions. Scheme 11 ...
... hydroborated a terminal olefin with dicylcohexylborane followed by oxidation to give gram quantities of the alcohol 27, a key intermediate in the synthesis of epothilone A (Scheme 11).26 Notice that the internal alkenyl group in 26 was not hydroborated under the reaction conditions. Scheme 11 ...
metal-catalyzed cross-coupling reactoins
... the substrate scope of cross-coupling reactions. Iron is also able to achieve comparable yields in alkylaryl and alkenyl-aryl cross-couplings, at lower temperatures and greatly reduced reaction times. However, iron is still limited by its dependence on the use of Grignard reagents. This has limited ...
... the substrate scope of cross-coupling reactions. Iron is also able to achieve comparable yields in alkylaryl and alkenyl-aryl cross-couplings, at lower temperatures and greatly reduced reaction times. However, iron is still limited by its dependence on the use of Grignard reagents. This has limited ...
Discodermolide

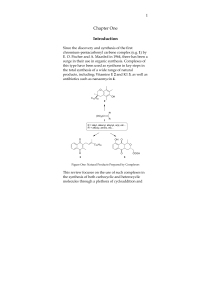
(+)-Discodermolide is a polyketide natural product found to stabilize microtubule. (+)-discodermolide was isolated by Gunasekera and his co-workers at the Harbor Branch Oceanographic Institute from the deep-sea sponge Discodermia dissoluta in 1990. (+)-Discodermolide was found to be a potent inhibitor of tumor cell growth in several MDR cancer cell lines. (+)-discodermolide also shows some unique characters, including a linear backbone structure, immunosuppressive properties both in vitro and in vivo, potent induction of an accelerated senescence phenotype, and synergistic antiproliferative activity in combination with paclitaxel. Discodermolide was recognized as one of the most potent natural promoters of tubulin assembly. A large number of efforts toward the total synthesis of (+)-discodermolide were directed by its interesting biological activities and extreme scarcity of natural sources (0.002% w/w from frozen marine sponge). The compound supply necessary for complete clinical trials cannot be met by harvesting, isolation, and purification. As of 2005, attempts at synthesis or semi-synthesis by fermentation have proven unsuccessful. As a result, all discodermolide used in preclinical studies and clinical trials has come from large-scale total synthesis.