CHE-310 Organic Chemistry I_
... For alkyl halides, alcohols and ethers, be able to name compounds correctly (nomenclature). Where necessay, be able to specify congiguration in the name. Know the two new mechanisms that we have learned in these chapters: SN2, SN1. Know which mechanisms go with which reactions under which conditions ...
... For alkyl halides, alcohols and ethers, be able to name compounds correctly (nomenclature). Where necessay, be able to specify congiguration in the name. Know the two new mechanisms that we have learned in these chapters: SN2, SN1. Know which mechanisms go with which reactions under which conditions ...
A theoretical study on the mechanisms of intermolecular
... an aldehyde with an alkene catalyzed by Wilkinson’s catalyst and an organic catalyst 2-amino-3-picoline in cationic and neutral systems. An aldehyde’s hydroacylation includes three stages: the C–H activation to form rhodium hydride (stage I), the alkene insertion into the Rh–H bond to give the Rh-al ...
... an aldehyde with an alkene catalyzed by Wilkinson’s catalyst and an organic catalyst 2-amino-3-picoline in cationic and neutral systems. An aldehyde’s hydroacylation includes three stages: the C–H activation to form rhodium hydride (stage I), the alkene insertion into the Rh–H bond to give the Rh-al ...
Grignard Reaction
... Despite more than a century of organic chemistry, the formation of C–C bonds is still one of the most difficult transformations that we encounter today. Fortunately, the ability of carbon to make bonds not only to C, H, N, O, S, and P (those elements found extensively in the living world), but also ...
... Despite more than a century of organic chemistry, the formation of C–C bonds is still one of the most difficult transformations that we encounter today. Fortunately, the ability of carbon to make bonds not only to C, H, N, O, S, and P (those elements found extensively in the living world), but also ...
friedel-craft reaction: a review - Advance Institute of Biotech
... Friedel–Crafts reaction is one of the oldest carbon–carbon bond forming processes, and is still an attractive method to introduce substituents on aromatic rings. This article mainly focused on Friedel–Crafts acylation, arylation , alkylation especially given by electron-rich arenes forming reaction ...
... Friedel–Crafts reaction is one of the oldest carbon–carbon bond forming processes, and is still an attractive method to introduce substituents on aromatic rings. This article mainly focused on Friedel–Crafts acylation, arylation , alkylation especially given by electron-rich arenes forming reaction ...
aryl halides
... sp2 hybridized and not prone to nucleophilic substitution. In a manner analogous to the phenols & alcohols, we have the same functional group in the two families, aryl halides and alkyl halides, but very different chemistries. ...
... sp2 hybridized and not prone to nucleophilic substitution. In a manner analogous to the phenols & alcohols, we have the same functional group in the two families, aryl halides and alkyl halides, but very different chemistries. ...
Ether C-O Bond Cleavage w
... Wenkert – Seminal Work •Extended π-system, e.g. naphthalene derivatives, afforded high yields with coupling at all the ...
... Wenkert – Seminal Work •Extended π-system, e.g. naphthalene derivatives, afforded high yields with coupling at all the ...
File
... hydrogen bonds with one another. This is why they boil at much lower temperatures than their isomeric alcohols Although ethers cannot form hydrogen bonds with one another, they do form hydrogen bonds with alcohols. This explains why ethers and alcohols are ...
... hydrogen bonds with one another. This is why they boil at much lower temperatures than their isomeric alcohols Although ethers cannot form hydrogen bonds with one another, they do form hydrogen bonds with alcohols. This explains why ethers and alcohols are ...
$doc.title
... Scheme 13: Classic Vedejs's synthesis of RBF3K salts ....................................................................17 Scheme 14: Decomposition of RBF3K salt and reformation of pinacol ester ................................18 Scheme ...
... Scheme 13: Classic Vedejs's synthesis of RBF3K salts ....................................................................17 Scheme 14: Decomposition of RBF3K salt and reformation of pinacol ester ................................18 Scheme ...
Chapter 1 Organoaluminum Reagents for Selective Organic
... [251] disclosed that three arene rings of ATPH form a propeller-like arrangement around the aluminum center, and hence ATPH has a cavity with C3 symmetry. By contrast, the X-ray crystal structure of the benzaldehyde-ATPH complex shows that the cavity surrounds the carbonyl substrate upon complexatio ...
... [251] disclosed that three arene rings of ATPH form a propeller-like arrangement around the aluminum center, and hence ATPH has a cavity with C3 symmetry. By contrast, the X-ray crystal structure of the benzaldehyde-ATPH complex shows that the cavity surrounds the carbonyl substrate upon complexatio ...
OxorheniumCatalyzed Deoxydehydration of Sugars and Sugar
... demand for sustainability.[1] However, one fundamental challenge is that saccharides, the major component of cellulosic biomass, are highly oxygen-rich when compared with the majority of current commodity chemicals and fuels. The polyol structure also generally presents poor solubility in organic so ...
... demand for sustainability.[1] However, one fundamental challenge is that saccharides, the major component of cellulosic biomass, are highly oxygen-rich when compared with the majority of current commodity chemicals and fuels. The polyol structure also generally presents poor solubility in organic so ...
pptx
... always perfect at stopping at the aldehyde oxidation state. The Swern oxidation is often a much cleaner reaction… this is what REAL organic chemists use! To carry out a Swern reaction: • Combine dimethyl sulfoxide (DMSO) and oxalyl chloride at –78 °C. • Add your alcohol, in solvent. Mix for awhile. ...
... always perfect at stopping at the aldehyde oxidation state. The Swern oxidation is often a much cleaner reaction… this is what REAL organic chemists use! To carry out a Swern reaction: • Combine dimethyl sulfoxide (DMSO) and oxalyl chloride at –78 °C. • Add your alcohol, in solvent. Mix for awhile. ...
Lecture8
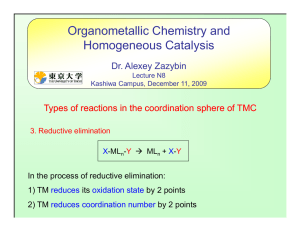
... elimination and (ii) the product, which has a lower coordination number, is stabilized • electron withdrawing ligands such as CO since these stabilize the low oxidation state (in the case of CO this is particularly effective because of π-backbonding). Similarly, if you want to design a complex that ...
... elimination and (ii) the product, which has a lower coordination number, is stabilized • electron withdrawing ligands such as CO since these stabilize the low oxidation state (in the case of CO this is particularly effective because of π-backbonding). Similarly, if you want to design a complex that ...
Topic Selection Menu - Pennsylvania State University
... Topic 15: Carboxylic Acids and Derivatives contd. • 15.2 Methanolysis of Acetyl Chloride – Nucleophilic acyl substitution mechanism – Influence of leaving group – Addition of nucleophile to Carbonyl ...
... Topic 15: Carboxylic Acids and Derivatives contd. • 15.2 Methanolysis of Acetyl Chloride – Nucleophilic acyl substitution mechanism – Influence of leaving group – Addition of nucleophile to Carbonyl ...
Topic Selection Menu - Pennsylvania State University
... Topic 14: Reactions of Aldehydes and Ketones contd. • 14.3 Hemiacetal-Acetal Formation • Hemiacetal formation mechanism ...
... Topic 14: Reactions of Aldehydes and Ketones contd. • 14.3 Hemiacetal-Acetal Formation • Hemiacetal formation mechanism ...
Review
... stabilizes the reaction quite a lot and allows it to happen faster. If the mechanism were SN2, the benzylic position wouldn’t be involved at all. If you do SN2 actually at the benzylic or allylic position, however, the reaction gets a lot faster. For allylic, it gets sped up by a couple of orders of ...
... stabilizes the reaction quite a lot and allows it to happen faster. If the mechanism were SN2, the benzylic position wouldn’t be involved at all. If you do SN2 actually at the benzylic or allylic position, however, the reaction gets a lot faster. For allylic, it gets sped up by a couple of orders of ...
Chapter 19 C-H Bond Activation with Neutral Platinum Methyl
... ligands undergo facile C-H bond activation reactions in non-coordinating solvents(6,7). While neutral platinum dimethyl complexes with these ligands could also be oxidized with dioxygen in methanol to give Pt(IV) alkyl products(8), cationic Pt methyl complexes derived from methane C-H bond activatio ...
... ligands undergo facile C-H bond activation reactions in non-coordinating solvents(6,7). While neutral platinum dimethyl complexes with these ligands could also be oxidized with dioxygen in methanol to give Pt(IV) alkyl products(8), cationic Pt methyl complexes derived from methane C-H bond activatio ...
Carbonyl Condensation Reactions
... conjugated enone as it is formed, especially if reaction conditions are pushed, eg high temperature. Mixed or "crossed" Aldol Reactions — If two different carbonyl compounds are allowed to react in an aldol reaction four products usually result; each carbonyl compound forms an enolate and each enola ...
... conjugated enone as it is formed, especially if reaction conditions are pushed, eg high temperature. Mixed or "crossed" Aldol Reactions — If two different carbonyl compounds are allowed to react in an aldol reaction four products usually result; each carbonyl compound forms an enolate and each enola ...
- Iranian Chemical Communication
... A general, mild and efficient protocol has been developed for the synthesis of esters and thioesters. The process has been taking place using tetra n-butylammonium iodide (TBAI) as a phase-transfer catalyst and in the presence of potassium carbonate (K2CO3). A wide range of esters and thioesters was ...
... A general, mild and efficient protocol has been developed for the synthesis of esters and thioesters. The process has been taking place using tetra n-butylammonium iodide (TBAI) as a phase-transfer catalyst and in the presence of potassium carbonate (K2CO3). A wide range of esters and thioesters was ...
Cl3CCN/PPh3 and CBr4/PPh3: two efficient reagent systems for the
... Br3CCOCBr3 were promising candidates in terms of new brominating agents for N-heteroaromatic hydroxy compounds, affording 2-bromopyridine in 15% and 45% yield, respectively (entries 2 and 3). Although, the use of Br3CCOCBr3 gave rise to the bromide in higher yield than CBr4, several by-products were ...
... Br3CCOCBr3 were promising candidates in terms of new brominating agents for N-heteroaromatic hydroxy compounds, affording 2-bromopyridine in 15% and 45% yield, respectively (entries 2 and 3). Although, the use of Br3CCOCBr3 gave rise to the bromide in higher yield than CBr4, several by-products were ...
Tunge - IARC Research
... standard asymmetric allylic alkylation using the palladium complex of Trost’s ligand 10 in both cases (Scheme 15). Since the allyl fragment is symmetrical, the same π-allyl complex is expected to form from either substrate. Stabilized nucleophiles like dimethyl malonate are known to attack the allyl ...
... standard asymmetric allylic alkylation using the palladium complex of Trost’s ligand 10 in both cases (Scheme 15). Since the allyl fragment is symmetrical, the same π-allyl complex is expected to form from either substrate. Stabilized nucleophiles like dimethyl malonate are known to attack the allyl ...
Palladium(II)-Catalyzed Oxidative Cyclization Strategies Andreas K. Å. Persson
... The use of transition metal catalysts has proven to be one of the most diverse tools for the mild and selective formation of carbon-carbon bonds. In particular palladium-catalyzed cross-coupling reactions have revolutionized the field. The main focus of this thesis has been directed towards preparat ...
... The use of transition metal catalysts has proven to be one of the most diverse tools for the mild and selective formation of carbon-carbon bonds. In particular palladium-catalyzed cross-coupling reactions have revolutionized the field. The main focus of this thesis has been directed towards preparat ...
I. ALDEHYDES AND KETONES Carbonyl compounds are
... most often used in addition reactions involving carbonyl compounds, as shown above. The product of these addition reactions is typically a secondary or tertiary alcohol (primary alcohols can be formed by reaction with formaldehyde), as shown in the examples below; in these the carbonyl and halide po ...
... most often used in addition reactions involving carbonyl compounds, as shown above. The product of these addition reactions is typically a secondary or tertiary alcohol (primary alcohols can be formed by reaction with formaldehyde), as shown in the examples below; in these the carbonyl and halide po ...
Amines - ChemConnections
... Question • Identify the product isolated from the reaction of p-nitroaniline with NaNO2 in H2SO4 followed by the addition of ...
... Question • Identify the product isolated from the reaction of p-nitroaniline with NaNO2 in H2SO4 followed by the addition of ...
CHM 3200 - Miami Dade College
... Fischer esterification, and conversion to acid chlorides). d. Identifying and illustrating reactions of acid/acyl chlorides (hydrolysis, esterification, and amidation). e. Identifying and illustrating reactions of esters and amides (reduction, hydrolysis, and saponification). Competency 11: The stud ...
... Fischer esterification, and conversion to acid chlorides). d. Identifying and illustrating reactions of acid/acyl chlorides (hydrolysis, esterification, and amidation). e. Identifying and illustrating reactions of esters and amides (reduction, hydrolysis, and saponification). Competency 11: The stud ...
Stille reaction

The Stille reaction, or the Migita-Kosugi-Stille coupling, is a chemical reaction widely used in organic synthesis which involves the coupling of an organotin compound (also known as organostannanes) with a variety of organic electrophiles via palladium-catalyzed coupling reaction.The R1 group attached to the trialkyltin is normally sp2-hybridized, including alkenes, and aryl groups; however, conditions have been devised to incorporate both sp3-hybridized groups, such as allylic and benzylic substituents, and sp-hybridized alkynes. These organostannanes are also stable to both air and moisture, and many of these reagents are either commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide, such as Cl, Br, I, yet pseudohalides such as triflates and sulfonates and phosphates can also be used.The groundwork for the Stille reaction was laid by Colin Eaborn, Toshihiko Migita, and Masanori Kosugi in 1976 and 1977, who explored numerous palladium catalyzed couplings involving organotin reagents. John Stille and David Milstein developed a much milder and more broadly applicable procedure in 1978. Stille’s work on this area might have earned him a share of the 2010 Nobel Prize, which was awarded to Richard Heck, Ei-ichi Negishi, and Akira Suzuki for their work on the Heck, Negishi, and Suzuki coupling reactions. However, Stille died in the plane crash of United Airlines Flight 232 in 1989.Several reviews have been published on the Stille reaction.