Triphase Catalysis. Applications to Organic Synthesis`
... Synthesis of Nitriles. Nucleophilic displacement by cyanide ion on organic halides represents the most commonly used method for the preparation of nitriles. Despite the usefulness of this approach, however, the required use of water and/or other polar and potentially nucleophilic solvents needed to ...
... Synthesis of Nitriles. Nucleophilic displacement by cyanide ion on organic halides represents the most commonly used method for the preparation of nitriles. Despite the usefulness of this approach, however, the required use of water and/or other polar and potentially nucleophilic solvents needed to ...
Copper perchlorate: Efficient acetylation catalyst
... achieved in contrast to some other catalysts. Acylal formation from aldehydes is found to be very rapid and at the same time keto groups remain unchanged. Thus the transition metal perclorates are better for acetylation hederoatoms and aldehydes than other metal perclorates and metal triflates. It m ...
... achieved in contrast to some other catalysts. Acylal formation from aldehydes is found to be very rapid and at the same time keto groups remain unchanged. Thus the transition metal perclorates are better for acetylation hederoatoms and aldehydes than other metal perclorates and metal triflates. It m ...
ppt
... 22.12: Reaction of Amines with Alkyl Halides. Amines react with alkyl halides and tosylates by nucleophilic substitution (SN2). Products from multiple alkylation often results. 22.13: The Hoffmann Elimination. 1° amine react with excess methyl iodide yield quarternary (4°) ammonium salts. E2 elimin ...
... 22.12: Reaction of Amines with Alkyl Halides. Amines react with alkyl halides and tosylates by nucleophilic substitution (SN2). Products from multiple alkylation often results. 22.13: The Hoffmann Elimination. 1° amine react with excess methyl iodide yield quarternary (4°) ammonium salts. E2 elimin ...
104 Chapter 22: Amines. Organic derivatives of ammonia, NH3
... 22.12: Reaction of Amines with Alkyl Halides. Amines react with alkyl halides and tosylates by nucleophilic substitution (SN2). Products from multiple alkylation often results. 22.13: The Hoffmann Elimination. 1° amine react with excess methyl iodide yield quarternary (4°) ammonium salts. E2 elimin ...
... 22.12: Reaction of Amines with Alkyl Halides. Amines react with alkyl halides and tosylates by nucleophilic substitution (SN2). Products from multiple alkylation often results. 22.13: The Hoffmann Elimination. 1° amine react with excess methyl iodide yield quarternary (4°) ammonium salts. E2 elimin ...
Montmorillonite: An efficient, heterogeneous and
... • May lower the activation energy of a reaction by stabilizing the transition state • May act as a general acid or base • Environmentally benign • Use of clays as catalysts allows for them to be recycled, which further increases their economic efficiency. • Furthermore, reactions that are catalyzed ...
... • May lower the activation energy of a reaction by stabilizing the transition state • May act as a general acid or base • Environmentally benign • Use of clays as catalysts allows for them to be recycled, which further increases their economic efficiency. • Furthermore, reactions that are catalyzed ...
Fluorination Chemistry - Sigma
... A new nucleophilic trifluoromethylation source was developed by the Colby group. The Colby Trifluoromethylation Reagent (L511315) is an air-stable solid that adds CF3 to ketones and thiols under mildly basic conditions. The major by-products from this reaction are DBU and trifluoroacetate, which are ...
... A new nucleophilic trifluoromethylation source was developed by the Colby group. The Colby Trifluoromethylation Reagent (L511315) is an air-stable solid that adds CF3 to ketones and thiols under mildly basic conditions. The major by-products from this reaction are DBU and trifluoroacetate, which are ...
Chapter 1 Introduction
... SalenH2 is commercially available. It was first prepared by Pfeiffer[1]. It is often generated in situ followed by the addition of the metal salt, but the ligand is also easily prepared as a pure organic compound ...
... SalenH2 is commercially available. It was first prepared by Pfeiffer[1]. It is often generated in situ followed by the addition of the metal salt, but the ligand is also easily prepared as a pure organic compound ...
Acid derivatives
... reactions take place. Indeed, an alert reader may well be puzzled by the facility of these nucleophilic substitution reactions. After all, it was previously noted that halogens bonded to sp2 or sp hybridized carbon atoms do not usually undergo substitution reactions with nucleophilic reagents. Furth ...
... reactions take place. Indeed, an alert reader may well be puzzled by the facility of these nucleophilic substitution reactions. After all, it was previously noted that halogens bonded to sp2 or sp hybridized carbon atoms do not usually undergo substitution reactions with nucleophilic reagents. Furth ...
a,b
... position of a ketone, ester, or nitrile by SN2 reaction of an enolate ion with an alkyl halide. • Look at the target molecule and identify any methyl or primary alkyl groups attached to an a carbon. • The target has an a methyl group, which might be introduced by alkylation of an ester enolate ion w ...
... position of a ketone, ester, or nitrile by SN2 reaction of an enolate ion with an alkyl halide. • Look at the target molecule and identify any methyl or primary alkyl groups attached to an a carbon. • The target has an a methyl group, which might be introduced by alkylation of an ester enolate ion w ...
Preparation of Cyclic Urethanes from Amino Alcohols and Carbon
... coordinated again by imidazolium cation to form a reactive intermediate 7 and this species could be transformed to the substituted cyclic urea thorough the formation of a cyclic intermediate 8 followed by dehydration. For 1c and 1d, the yields of cyclic urea are smaller compared with 1a (Fig. 4); fo ...
... coordinated again by imidazolium cation to form a reactive intermediate 7 and this species could be transformed to the substituted cyclic urea thorough the formation of a cyclic intermediate 8 followed by dehydration. For 1c and 1d, the yields of cyclic urea are smaller compared with 1a (Fig. 4); fo ...
CHAPTER 17: Carbonyl group (1)
... An alternative method is the PCC oxidation (CrO3, HCl, pyridine) that oxidizes primary and secondary alcohols only to the corresponding carbonyl groups (no overoxidation to acids). The PCC oxidation is performed in organic solvents, such as CH2Cl2. In the absense of H2O the primary aldehyde cannot b ...
... An alternative method is the PCC oxidation (CrO3, HCl, pyridine) that oxidizes primary and secondary alcohols only to the corresponding carbonyl groups (no overoxidation to acids). The PCC oxidation is performed in organic solvents, such as CH2Cl2. In the absense of H2O the primary aldehyde cannot b ...
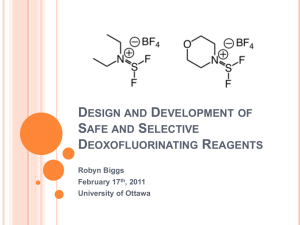
Design and Development of Safe and Selective Deoxofluorinating
... Umemoto, T.; Singh, R. P.; Xu, Y.; Saito, N. J. Am. Chem. Soc. 2010, 132, 18199. ...
... Umemoto, T.; Singh, R. P.; Xu, Y.; Saito, N. J. Am. Chem. Soc. 2010, 132, 18199. ...
more aromatic chemistry
... conditions that reduce alkene double bonds Can selectively reduce an alkene double bond in the presence of an aromatic ring Reduction of an aromatic ring requires more powerful reducing conditions (high pressure or rhodium catalysts) ...
... conditions that reduce alkene double bonds Can selectively reduce an alkene double bond in the presence of an aromatic ring Reduction of an aromatic ring requires more powerful reducing conditions (high pressure or rhodium catalysts) ...
enzymatic And Limited Industrial Use
... A reaction that can proceed in more than one way to produce different products involving different carbon atoms, where one predominates. It is said to be regioselective. ...
... A reaction that can proceed in more than one way to produce different products involving different carbon atoms, where one predominates. It is said to be regioselective. ...
Two New Ruthenium(II) Complexes with Cyclometalated 2
... also of a second compound which later was identified as the mononuclear complex 2 by an independent synthesis (vide infra). Under the optimized reaction conditions mentioned above, only traces of 2 and Ru3 (CO)12 were detected when the reaction was stopped. The mononuclear complex 2 could be prepare ...
... also of a second compound which later was identified as the mononuclear complex 2 by an independent synthesis (vide infra). Under the optimized reaction conditions mentioned above, only traces of 2 and Ru3 (CO)12 were detected when the reaction was stopped. The mononuclear complex 2 could be prepare ...
Chapter 19. Aldehydes and Ketones
... addition forming the conjugate acid of C=O Addition yields a hydroxy ether, called a hemiacetal (reversible); further reaction can occur Protonation of the –OH and loss of water leads to an oxonium ion, R2C=OR+ to which a second alcohol adds to form the acetal ...
... addition forming the conjugate acid of C=O Addition yields a hydroxy ether, called a hemiacetal (reversible); further reaction can occur Protonation of the –OH and loss of water leads to an oxonium ion, R2C=OR+ to which a second alcohol adds to form the acetal ...
Lecture notes for chapter 6
... higher metals with a d6 or d8 electron count (sometimes d4). 4) Schrock alkylidenes as dianionic 4 e- donor ligands. Typically group 4 or 5 metals with d0 electron counts. Also later transition metals in high oxidation states (d0, d2, or d4). Of course, in order to do method 3 or 4, you have to real ...
... higher metals with a d6 or d8 electron count (sometimes d4). 4) Schrock alkylidenes as dianionic 4 e- donor ligands. Typically group 4 or 5 metals with d0 electron counts. Also later transition metals in high oxidation states (d0, d2, or d4). Of course, in order to do method 3 or 4, you have to real ...
Zinc Alkyls in Organic Synthesis
... Asymmetric Additions to Aldehydes and Ketones DEZ is also useful in catalytic asymmetric addition to aldehydes or ketones forming chiral secondary or tertiary alcohols. These reactions typically involve an amine or a sulfonamide ligand in combination with tetraisopropyl titanate (TIPT). The added s ...
... Asymmetric Additions to Aldehydes and Ketones DEZ is also useful in catalytic asymmetric addition to aldehydes or ketones forming chiral secondary or tertiary alcohols. These reactions typically involve an amine or a sulfonamide ligand in combination with tetraisopropyl titanate (TIPT). The added s ...
Chemistry 0310 - Organic Chemistry 1 Chapter 12. Reactions of
... Chapter 12. Reactions of Alkenes ...
... Chapter 12. Reactions of Alkenes ...
Synthesis of Alcohols Using Grignard Reagents Grignard reagents
... CH3(CH2)3C CCH2OH other than a Grignard? Grignard? ...
... CH3(CH2)3C CCH2OH other than a Grignard? Grignard? ...
Triruthenium and triosmium carbonyl clusters containing chiral
... were assigned on the basis of TLC elution rates. Using the same eluent, compounds 5 and 7 moved on their TLC plates faster than their diastereoisomers 6 and 8, respectively. Consequently, we assigned to the osmium compounds 7 and 8 the same structures as the those of the ruthenium complexes 5 and 6, ...
... were assigned on the basis of TLC elution rates. Using the same eluent, compounds 5 and 7 moved on their TLC plates faster than their diastereoisomers 6 and 8, respectively. Consequently, we assigned to the osmium compounds 7 and 8 the same structures as the those of the ruthenium complexes 5 and 6, ...
Document
... synthesis. A number of methods using various reagents have been reported to bring about this transformation (Scheme 1).1-9 However, many of these procedures suffer from several limitations such as involvement of toxic heavy metal oxidants, expensive catalyst, large excess of reagents, and dry solven ...
... synthesis. A number of methods using various reagents have been reported to bring about this transformation (Scheme 1).1-9 However, many of these procedures suffer from several limitations such as involvement of toxic heavy metal oxidants, expensive catalyst, large excess of reagents, and dry solven ...
ether - HCC Southeast Commons
... are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal reactivity? ...
... are prepared by the sulfuric acid-catalyzed dehydration procedure? What product(s) would you expect if ethanol and 1-propanol were allowed to react together? In what ratio would the products be formed if the two alcohols were of equal reactivity? ...
Review on N acylation reaction
... Thionyl chloride remains the most popular reagent of preparation of acid chlorides with advantages while some disadvantages which can be avoided by using recently developed reagents when required. Thionyl chloride is volatile and excess can be distilled off at the end, leaving acid chloride. Only ga ...
... Thionyl chloride remains the most popular reagent of preparation of acid chlorides with advantages while some disadvantages which can be avoided by using recently developed reagents when required. Thionyl chloride is volatile and excess can be distilled off at the end, leaving acid chloride. Only ga ...
Stille reaction

The Stille reaction, or the Migita-Kosugi-Stille coupling, is a chemical reaction widely used in organic synthesis which involves the coupling of an organotin compound (also known as organostannanes) with a variety of organic electrophiles via palladium-catalyzed coupling reaction.The R1 group attached to the trialkyltin is normally sp2-hybridized, including alkenes, and aryl groups; however, conditions have been devised to incorporate both sp3-hybridized groups, such as allylic and benzylic substituents, and sp-hybridized alkynes. These organostannanes are also stable to both air and moisture, and many of these reagents are either commercially available or can be synthesized from literature precedent. However, these tin reagents tend to be highly toxic. X is typically a halide, such as Cl, Br, I, yet pseudohalides such as triflates and sulfonates and phosphates can also be used.The groundwork for the Stille reaction was laid by Colin Eaborn, Toshihiko Migita, and Masanori Kosugi in 1976 and 1977, who explored numerous palladium catalyzed couplings involving organotin reagents. John Stille and David Milstein developed a much milder and more broadly applicable procedure in 1978. Stille’s work on this area might have earned him a share of the 2010 Nobel Prize, which was awarded to Richard Heck, Ei-ichi Negishi, and Akira Suzuki for their work on the Heck, Negishi, and Suzuki coupling reactions. However, Stille died in the plane crash of United Airlines Flight 232 in 1989.Several reviews have been published on the Stille reaction.