chemistry_23 - Bonar Law Memorial
... a class of chemical compounds called ethers. You will read about the chemical characteristics of ethers that make them good anesthetics. Alcohols How are alcohols classified and named? • An alcohol is an organic compound with an — OH group. • The —OH functional group in alcohols is called a hydroxyl ...
... a class of chemical compounds called ethers. You will read about the chemical characteristics of ethers that make them good anesthetics. Alcohols How are alcohols classified and named? • An alcohol is an organic compound with an — OH group. • The —OH functional group in alcohols is called a hydroxyl ...
Worksheet 10.1
... Alkene Unsaturated hydrocarbons. They contain a carbon–carbon double bond. They have the general formula CnH2n. Amine A homologous series containing the functional group –NH2. Secondary and tertiary amines respectively have one or two of the hydrogen atoms substituted by alkyl groups. Carbocation A ...
... Alkene Unsaturated hydrocarbons. They contain a carbon–carbon double bond. They have the general formula CnH2n. Amine A homologous series containing the functional group –NH2. Secondary and tertiary amines respectively have one or two of the hydrogen atoms substituted by alkyl groups. Carbocation A ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
organic chemistry i
... Chlorination: a substitution reaction Control of chlorination Reaction with other halogens: halogenation Relative reactivity Reaction mechanisms Mechanism of chlorination. Free radicals Chain reactions Inhibitors Heat of reaction Energy of activation ...
... Chlorination: a substitution reaction Control of chlorination Reaction with other halogens: halogenation Relative reactivity Reaction mechanisms Mechanism of chlorination. Free radicals Chain reactions Inhibitors Heat of reaction Energy of activation ...
CfE Higher Chemistry Homework Unit 2: Natures Chemistry Soaps
... What term can be applied to aspirin but not oil of wintergreen? ...
... What term can be applied to aspirin but not oil of wintergreen? ...
Soaps, Fragrances and Skin Care 1. In which line of the table are fat
... What term can be applied to aspirin but not oil of wintergreen? 4. A student carried out four tests on ethanol and ethanoic acids to compare the properties of the two homologous series, alcohols and carboxylic acids. a. Choose one test in which ethanol and ethanoic acid will give different results a ...
... What term can be applied to aspirin but not oil of wintergreen? 4. A student carried out four tests on ethanol and ethanoic acids to compare the properties of the two homologous series, alcohols and carboxylic acids. a. Choose one test in which ethanol and ethanoic acid will give different results a ...
Word - chemmybear.com
... b) the molecule has some polarity c) they ionize completely in water solution ...
... b) the molecule has some polarity c) they ionize completely in water solution ...
Acrobat - chemmybear.com
... b) the molecule has some polarity c) they ionize completely in water solution ...
... b) the molecule has some polarity c) they ionize completely in water solution ...
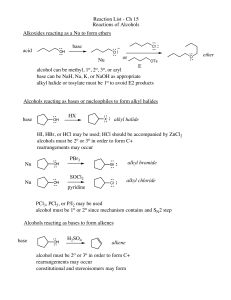
Reaction List - Ch 15 Reactions of Alcohols Alkoxides reacting as a
... base can be NaH, Na, K, or NaOH as appropriate alkyl halide or tosylate must be 1o to avoid E2 products Alcohols reacting as bases or nucleophiles to form alkyl halides base ...
... base can be NaH, Na, K, or NaOH as appropriate alkyl halide or tosylate must be 1o to avoid E2 products Alcohols reacting as bases or nucleophiles to form alkyl halides base ...
2010-09-16 Alcohols
... Classification of alcohols: Alcohols are categorized based on the type of carbon that they are attached to. Since carbon atoms have 4 bonds, the atom that has the OH can be attached to 1,2 or 3 alkyl groups as well. This creates primary, secondary and tertiary alcohols respectively. The classificat ...
... Classification of alcohols: Alcohols are categorized based on the type of carbon that they are attached to. Since carbon atoms have 4 bonds, the atom that has the OH can be attached to 1,2 or 3 alkyl groups as well. This creates primary, secondary and tertiary alcohols respectively. The classificat ...
hydrocarbons summary
... There are common names for many organic compounds. For example: methyl alcohol, acetylene, acetic acid, etc. Yet, there are an infinite number of possible organic structures. Thus, it is important to name them in a systematic way. The purpose of IUPAC names is to provide a set of rules so that the s ...
... There are common names for many organic compounds. For example: methyl alcohol, acetylene, acetic acid, etc. Yet, there are an infinite number of possible organic structures. Thus, it is important to name them in a systematic way. The purpose of IUPAC names is to provide a set of rules so that the s ...
organic chemistry - Turner Fenton Secondary School
... ALCOHOL NOMENCLATURE Alcohols are compounds that contain the functional group –OH (Hydroxyl group). This hydroxyl group can be attached to one, or two or even three alkyl groups. The short way of representing an alkyl group is using R. Therefore: an alcohol can generally be represented at R-OH Rec ...
... ALCOHOL NOMENCLATURE Alcohols are compounds that contain the functional group –OH (Hydroxyl group). This hydroxyl group can be attached to one, or two or even three alkyl groups. The short way of representing an alkyl group is using R. Therefore: an alcohol can generally be represented at R-OH Rec ...
Study Guide on Ch 5 and 6
... L. Glycocidic Bonds (p. 236) a. Used to link another sugar molecule. b. (1→4) glycocidic bond c. (1→4) glycocidic bond ...
... L. Glycocidic Bonds (p. 236) a. Used to link another sugar molecule. b. (1→4) glycocidic bond c. (1→4) glycocidic bond ...
Guideline
... carbocation. This rule does not hold for simple alkenes because of the ready migration of the double bond, but it does hold for cycloalkanes. 6. Saturated rings tend to lose alkyl side chains at the alpha bond. This is merely a special case of branching. The positive charge tends to stay with the ri ...
... carbocation. This rule does not hold for simple alkenes because of the ready migration of the double bond, but it does hold for cycloalkanes. 6. Saturated rings tend to lose alkyl side chains at the alpha bond. This is merely a special case of branching. The positive charge tends to stay with the ri ...
Exam 2 Review A
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
... You should be familiar with the detailed mechanisms of the SN1 and SN2 reactions. Rate determining steps are important to consider, as are the transition states associated with these steps. Compare and contrast the SN1 and SN2 reactions with respect to kinetics, nature of the electrophile [structure ...
Types of Reactions in Organic Chemistry Chemistry
... takes place when methane reacts with chlorine. A chlorine atom has replaced a hydrogen atom in a molecule of methane. This is known as halogenation of alkanes or more specifically the chlorination of methane is referred to as a free radical substitution reaction and it involves four steps: initiatio ...
... takes place when methane reacts with chlorine. A chlorine atom has replaced a hydrogen atom in a molecule of methane. This is known as halogenation of alkanes or more specifically the chlorination of methane is referred to as a free radical substitution reaction and it involves four steps: initiatio ...
Chapter 7
... The greater the number of attached alkyl groups The more highly substituted the carbon atoms of the double bond) the greater the alkene’s stability ...
... The greater the number of attached alkyl groups The more highly substituted the carbon atoms of the double bond) the greater the alkene’s stability ...
Hydrocarbon Derivatives:
... • adding functional group changes chemical & physical properties of molecule in specific ways – changes depend on type functional group added ...
... • adding functional group changes chemical & physical properties of molecule in specific ways – changes depend on type functional group added ...
CHAPTER 1: ORGANIC COMPOUNDS
... 5. don’t dissolve in polar solvents such as water 1.3: Reactions of Hydrocarbons - all burn to give CO2, H2O and huge amounts of heat energy - less reactive - Alkanes aromatic compounds alkenes alkynes – more reactive Reactions of Alkanes: - single C-C bonds stable and so unreactive, generally ...
... 5. don’t dissolve in polar solvents such as water 1.3: Reactions of Hydrocarbons - all burn to give CO2, H2O and huge amounts of heat energy - less reactive - Alkanes aromatic compounds alkenes alkynes – more reactive Reactions of Alkanes: - single C-C bonds stable and so unreactive, generally ...
Oxacyclopropane (Epoxide) Synthesis: Epoxidation by
... In the presence of oxygen, a radical chain sequence mechanism leads to the antiMarkovnikov product. Small amounts of peroxides (RO-OR) are formed in alkene samples stored in the presence of air (O2). The peroxides initiate the radical chain sequence mechanism, which is much faster than the ionic me ...
... In the presence of oxygen, a radical chain sequence mechanism leads to the antiMarkovnikov product. Small amounts of peroxides (RO-OR) are formed in alkene samples stored in the presence of air (O2). The peroxides initiate the radical chain sequence mechanism, which is much faster than the ionic me ...
Organic Synthesis
... properties of compounds are matters of simple observation. Synthesis, though, has some science and some art to it. There is rarely a ‘right’ answer. Most synthetic problems have more than one solution, and the trick is to judge which of these is likely to have the best chance of success. Even the mo ...
... properties of compounds are matters of simple observation. Synthesis, though, has some science and some art to it. There is rarely a ‘right’ answer. Most synthetic problems have more than one solution, and the trick is to judge which of these is likely to have the best chance of success. Even the mo ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.