Functional Groups and nomenclature Major concepts Stable
... long chain. What functional group(s) are in the monomer? In the polymer? Based on this information, what general class of polymers does PHB belong to? (Hint: popular clothing material of the 1970’s.) What environmentally friendly small molecule is the side-product of this reaction? (Look at the atom ...
... long chain. What functional group(s) are in the monomer? In the polymer? Based on this information, what general class of polymers does PHB belong to? (Hint: popular clothing material of the 1970’s.) What environmentally friendly small molecule is the side-product of this reaction? (Look at the atom ...
Organic Chemistry Notes
... petrol mix – a high octane number of 100 burns very ____________ while a low octane number burns ____________. A petrol with an octane number of 94 indicates a mixture that burns with the same characteristics as a mixture of 94 parts heptane and 7 parts 2, 2, 4 – trimethylpentane. Typical petrol has ...
... petrol mix – a high octane number of 100 burns very ____________ while a low octane number burns ____________. A petrol with an octane number of 94 indicates a mixture that burns with the same characteristics as a mixture of 94 parts heptane and 7 parts 2, 2, 4 – trimethylpentane. Typical petrol has ...
Full Unit Summary Booklet
... Coal, oil and natural gas are fossil fuels. Fossil fuels are described as a finite resource as they will eventually run out. Fossil fuels are formed over millions of years. They are the fossilised remains of dead plants and animals. Plants and animals died and fell to the sea or swamp floor and were ...
... Coal, oil and natural gas are fossil fuels. Fossil fuels are described as a finite resource as they will eventually run out. Fossil fuels are formed over millions of years. They are the fossilised remains of dead plants and animals. Plants and animals died and fell to the sea or swamp floor and were ...
This is the first exam with targeted syntheses that you
... Most of the reactions of aldehydes and ketones in these chapters are nucleophilic addition reactions. The oxygen in C=O polarizes the bond. Therefore, while electrophilic addition (electrophile first, followed by nucleophile) was favored for the comparatively non-polar, electron-rich alkene, carbony ...
... Most of the reactions of aldehydes and ketones in these chapters are nucleophilic addition reactions. The oxygen in C=O polarizes the bond. Therefore, while electrophilic addition (electrophile first, followed by nucleophile) was favored for the comparatively non-polar, electron-rich alkene, carbony ...
reactions of alcohols
... Summary of Oxidation reactions of the alcohols • potassium dichromate K2Cr2O7 is an oxidising agent that causes alcohols to oxidise. • When it reacts it changes from orange to green The exact reaction, however, depends on the type of alcohol i.e. whether it is primary, secondary, or tertiary, and on ...
... Summary of Oxidation reactions of the alcohols • potassium dichromate K2Cr2O7 is an oxidising agent that causes alcohols to oxidise. • When it reacts it changes from orange to green The exact reaction, however, depends on the type of alcohol i.e. whether it is primary, secondary, or tertiary, and on ...
Chemical Reactions
... will also often produce a precipitate (solid),a gas, or some molecular compound such as water. ...
... will also often produce a precipitate (solid),a gas, or some molecular compound such as water. ...
Formal Charge
... the formal charge, if any, borne by the atom. Bonds to atoms with equal electronegativity (i.e., bonds to another atom of the same element) need not be considered. Only F is more electronegative than O so NO is always –2 unless an F-O bond is present. In commonly encountered organic compounds, only ...
... the formal charge, if any, borne by the atom. Bonds to atoms with equal electronegativity (i.e., bonds to another atom of the same element) need not be considered. Only F is more electronegative than O so NO is always –2 unless an F-O bond is present. In commonly encountered organic compounds, only ...
Group 13 Compounds - University of Ottawa
... group, thus hydroboration is the most important in synthetic chemistry. A possible explanation is that the orbitals involved are more extensive as n increases, making the bond distance longer. Thus the boron is more responsive to the steric environment than the rest of group 13. As well, hyperconjug ...
... group, thus hydroboration is the most important in synthetic chemistry. A possible explanation is that the orbitals involved are more extensive as n increases, making the bond distance longer. Thus the boron is more responsive to the steric environment than the rest of group 13. As well, hyperconjug ...
reactions taking place within cells
... More substitutions Dependent on amount of excess chlorine or methane Excess chlorine Cl• free radicals start attacking chloromethane giving dichloromethane CH 2Cl2 trichloromethane CHCl3 tetrachloromethane CCl4 Excess methane Product will be mostly chloromethane Alkenes CnH2n Unsaturated molecules b ...
... More substitutions Dependent on amount of excess chlorine or methane Excess chlorine Cl• free radicals start attacking chloromethane giving dichloromethane CH 2Cl2 trichloromethane CHCl3 tetrachloromethane CCl4 Excess methane Product will be mostly chloromethane Alkenes CnH2n Unsaturated molecules b ...
Chemistry: Selected Topics
... The aim of the course is to acquaint the students with the properties and synthesis of some important types of chemical compounds. In the first part of the course, the general concepts of chemical reaction kinetics are presented with emphasis on the relation between reaction rate and reaction mechan ...
... The aim of the course is to acquaint the students with the properties and synthesis of some important types of chemical compounds. In the first part of the course, the general concepts of chemical reaction kinetics are presented with emphasis on the relation between reaction rate and reaction mechan ...
Organic Chemistry 2014 finalzzz
... Number the carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (use the suffix –yl for branches) Write the complete IUPAC name, following the format: (number of location, if nece ...
... Number the carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (use the suffix –yl for branches) Write the complete IUPAC name, following the format: (number of location, if nece ...
2.7 INTRODUCTION TO FUNCTIONAL GROUPS
... can anyone learn the chemistry of all of them? Fortunately, we do not need to learn an entire new set of chemical reactions for each new compound encountered. A particular arrangement or group of atoms has very similar chemistry no matter what the remainder of the molecule looks like. Let’s consider ...
... can anyone learn the chemistry of all of them? Fortunately, we do not need to learn an entire new set of chemical reactions for each new compound encountered. A particular arrangement or group of atoms has very similar chemistry no matter what the remainder of the molecule looks like. Let’s consider ...
Atomic Structure (27 Jan 2004) • What is matter? • Dalton`s Atomic
... saturated and unsaturated hydrocarbons • Petroleum: separating fractions from crude oil • Catalytic cracking and reforming • Fuel and octane rating ...
... saturated and unsaturated hydrocarbons • Petroleum: separating fractions from crude oil • Catalytic cracking and reforming • Fuel and octane rating ...
كيمياء عضويةc - جامعة دمنهور
... Differentiate the carbonyl group in aldehydes and ketones, and hydroxyl group in alcohols and phenols. Correlate between structure of water with alcohols, phenols, and ethers. Compare the effect of alkyl and aryl groups on the chemistry of characteristics functional groups. Define the electrophilic ...
... Differentiate the carbonyl group in aldehydes and ketones, and hydroxyl group in alcohols and phenols. Correlate between structure of water with alcohols, phenols, and ethers. Compare the effect of alkyl and aryl groups on the chemistry of characteristics functional groups. Define the electrophilic ...
2287 Summary
... Brominated Ketones.-The procedure for the preparation was that of Schmidt,6 according to which the ketone is brominated in glacial acetic acid. a-Bromo-n-va1erophenone.-As this substance has not previously been described in the literature, it may be mentioned that it is a straw-colored liquid boilin ...
... Brominated Ketones.-The procedure for the preparation was that of Schmidt,6 according to which the ketone is brominated in glacial acetic acid. a-Bromo-n-va1erophenone.-As this substance has not previously been described in the literature, it may be mentioned that it is a straw-colored liquid boilin ...
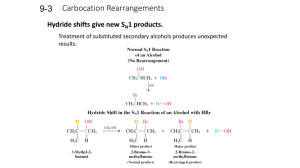
Carbocation Rearrangements
... Alkyl and hydride shifts to primary carbons bearing leaving groups can occur without the formation of primary carbocations. ...
... Alkyl and hydride shifts to primary carbons bearing leaving groups can occur without the formation of primary carbocations. ...
Module - EPS School Projects - Heriot
... provide a range of methods for the interconversion of key functional groups, emphasising issues of regio- and stereocontrol discuss an extended range of reactions for the formation of carbon-carbon bonds develop the concept of retrosynthetic analysis (RSA) in a structured manner illustrate h ...
... provide a range of methods for the interconversion of key functional groups, emphasising issues of regio- and stereocontrol discuss an extended range of reactions for the formation of carbon-carbon bonds develop the concept of retrosynthetic analysis (RSA) in a structured manner illustrate h ...
OrganicChemistrySV
... that is attached to two other carbon atoms - named by replacing the final –e from the corresponding alkane with –one; if necessary, cite which carbon atom the carbonyl group is attached to. ...
... that is attached to two other carbon atoms - named by replacing the final –e from the corresponding alkane with –one; if necessary, cite which carbon atom the carbonyl group is attached to. ...
Organic Chemistry = ______________________ ________________________
... that is attached to two other carbon atoms - named by replacing the final –e from the corresponding alkane with –one; if necessary, cite which carbon atom the carbonyl group is attached to. ...
... that is attached to two other carbon atoms - named by replacing the final –e from the corresponding alkane with –one; if necessary, cite which carbon atom the carbonyl group is attached to. ...
Chapter 16 Alkanes and alkenes
... example: vegetable oils are polyunsaturated oils manufacture of margarine by addition reaction; vegetable oil reacts with hydrogen in presence of nickel catalyst; H2 molecules add to the C=C bonds of vegetable oil after addition reaction, vegetable oil has more hydrogen atoms and hence, greate ...
... example: vegetable oils are polyunsaturated oils manufacture of margarine by addition reaction; vegetable oil reacts with hydrogen in presence of nickel catalyst; H2 molecules add to the C=C bonds of vegetable oil after addition reaction, vegetable oil has more hydrogen atoms and hence, greate ...
幻灯片 1 - Sun Yat-sen University
... 3. Coolants (CCl2F2 = Freon). The widespread use of chlorofluorocarbons is now thought to be one of the major causes for decrease in the ozone layer. Carbon tetrachloride is a reagent in synthetic chemistry and was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a ...
... 3. Coolants (CCl2F2 = Freon). The widespread use of chlorofluorocarbons is now thought to be one of the major causes for decrease in the ozone layer. Carbon tetrachloride is a reagent in synthetic chemistry and was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a ...
Electophilic Aromatic Substituion
... Alkali Fusion of Aromatic Sulfonic Acids • Sulfonic acids are useful as intermediates • Heating with NaOH at 300 ºC followed by neutralization with acid replaces the SO3H group with an OH • Example is the synthesis of p-cresol ...
... Alkali Fusion of Aromatic Sulfonic Acids • Sulfonic acids are useful as intermediates • Heating with NaOH at 300 ºC followed by neutralization with acid replaces the SO3H group with an OH • Example is the synthesis of p-cresol ...
Chemistry 322 Experiment #3 Data Sheet
... 4. Flow Chart. Fill in the empty rectangles with the appropriate cations. In the empty ovals, fill in the appropriate methylpentene structures. H+ OH ...
... 4. Flow Chart. Fill in the empty rectangles with the appropriate cations. In the empty ovals, fill in the appropriate methylpentene structures. H+ OH ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.