17.2.3 Interhalogen compounds(65-67)
... is quite different, being built up of planar 12Cls molecules separated by normal van der Waals' distances between the Cl atoms (Fig. 17.7~).The terminal I-CI distances are similar to those in IC1 but the bridging I-CI distances are appreciably ...
... is quite different, being built up of planar 12Cls molecules separated by normal van der Waals' distances between the Cl atoms (Fig. 17.7~).The terminal I-CI distances are similar to those in IC1 but the bridging I-CI distances are appreciably ...
Full-Text PDF
... Abstract: Density functional theory (DFT) calculations have been used to describe the first turnover of an olefin metathesis reaction calling for a new in silico family of homogenous Ru-based catalysts bearing a phosphine–phosphonium ylide ligand, with ethylene as a substrate. Equal to conventional ...
... Abstract: Density functional theory (DFT) calculations have been used to describe the first turnover of an olefin metathesis reaction calling for a new in silico family of homogenous Ru-based catalysts bearing a phosphine–phosphonium ylide ligand, with ethylene as a substrate. Equal to conventional ...
Chemistry - Gildredge House
... The course is designed to provide students with essential knowledge and understanding of different areas of Chemistry and how they relate to each other. Students gain essential practical skills as well as a deep knowledge and understanding of scientific methods and competence in a variety of mathema ...
... The course is designed to provide students with essential knowledge and understanding of different areas of Chemistry and how they relate to each other. Students gain essential practical skills as well as a deep knowledge and understanding of scientific methods and competence in a variety of mathema ...
PPT Oxidation
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
PPT Oxidation
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
... reduced and get oxidized. Here are the two halfreactions from the example: Ag+ ---> Ag Cu ---> Cu2+ • The silver is being reduced, its oxidation number going from +1 to zero. The copper's oxidation number went from zero to +2, so it was oxidized in the reaction. In order to figure out the halfreacti ...
Synthesis and characterization of glycoconjugate tin(IV) complexes
... The human tumor cell lines used for in vitro screening for antitumor activity were Hop62 and A549 (lung), PC3 and DU145 (prostate), A498 (kidney), DWD (oral), Colo205, HT29 HCT15 and SW620 (colon) T24 (bladder) MIA-PA-CA-2 (pancreas), MCF7 and ZR-75-1 (breast), SiHa, ME180 and HeLa (cervix) U373MG ( ...
... The human tumor cell lines used for in vitro screening for antitumor activity were Hop62 and A549 (lung), PC3 and DU145 (prostate), A498 (kidney), DWD (oral), Colo205, HT29 HCT15 and SW620 (colon) T24 (bladder) MIA-PA-CA-2 (pancreas), MCF7 and ZR-75-1 (breast), SiHa, ME180 and HeLa (cervix) U373MG ( ...
Nanostructured Transition Metal Chalcogenides as - KIT
... Transition metal sulfides like CuS were initially successfully used as lithium primary cell materials.[2] In the 70s some researcher realized that layered phases like TiS2 and MoS2 were also candidates as positive electrode materials for rechargeable lithium batteries.[3,4] Whittingham was the first ...
... Transition metal sulfides like CuS were initially successfully used as lithium primary cell materials.[2] In the 70s some researcher realized that layered phases like TiS2 and MoS2 were also candidates as positive electrode materials for rechargeable lithium batteries.[3,4] Whittingham was the first ...
Chapter 2
... The Atomic Theory of Matter Dalton’s Theory of Matter: 1. Each element is composed of extremely small particles call atoms. 2. All atoms of a given element are identical; atoms of different elements are different. 3. Atoms of an element are not changed into different types of atoms by a chemical re ...
... The Atomic Theory of Matter Dalton’s Theory of Matter: 1. Each element is composed of extremely small particles call atoms. 2. All atoms of a given element are identical; atoms of different elements are different. 3. Atoms of an element are not changed into different types of atoms by a chemical re ...
Inorganic and organic chemistry 2
... The coordination number is the number of coordinate bonds between the ligand(s) and the central metal atom or ion. EDTA4− is a hexadentate ligand, so one ion forms six coordinate bonds. The other ligands are monodentate so each forms a single coordinate bond with the central metal atom or ion. ...
... The coordination number is the number of coordinate bonds between the ligand(s) and the central metal atom or ion. EDTA4− is a hexadentate ligand, so one ion forms six coordinate bonds. The other ligands are monodentate so each forms a single coordinate bond with the central metal atom or ion. ...
Chapter 2 Expanded Notes
... becomes negatively charged. If it loses electrons it becomes positively charged. Note: although the formation of ions is necessary to form ionic bonds, it is not a guarantee that a bond will form. Ion = an atom or molecule with an electrical charge resulting from the gain or loss of 1 or more electr ...
... becomes negatively charged. If it loses electrons it becomes positively charged. Note: although the formation of ions is necessary to form ionic bonds, it is not a guarantee that a bond will form. Ion = an atom or molecule with an electrical charge resulting from the gain or loss of 1 or more electr ...
Metal-directed self-assembly has been widely used to design and to
... Metal-directed self-assembly has been widely used to design and to construct finite1 and infinite2-9 networks that have many potential applications in magnetics, optics, catalysis, molecular recognition and separations. The vast majority of reported compounds are metal-organic networks, with little ...
... Metal-directed self-assembly has been widely used to design and to construct finite1 and infinite2-9 networks that have many potential applications in magnetics, optics, catalysis, molecular recognition and separations. The vast majority of reported compounds are metal-organic networks, with little ...
Net ionic equation
... Reactions of acids and bases •Neutralization: acid + base are mixed: HNO3(aq) + KOH(aq) ??? •Salt = ionic compound cation from base anion from acid. •Neutralization of acid with metal hydroxide produces water and a salt. •Acids + carbonates = CO2 and H2O ...
... Reactions of acids and bases •Neutralization: acid + base are mixed: HNO3(aq) + KOH(aq) ??? •Salt = ionic compound cation from base anion from acid. •Neutralization of acid with metal hydroxide produces water and a salt. •Acids + carbonates = CO2 and H2O ...
Name: (1 of 2) Math Set # 13 Protons, Neutrons, Electrons Proton
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
... An ionic bond is created between metals and nonmetals. This is because a metal in group 1 or 2 gives up electrons easily and nonmetals in groups 16 through 18 accept electrons easily. An ionic bond results in two or more ions being attracted to each other. The total charge of the molecule must be ze ...
Lecture 2
... or cations with d electrons not available for π-bonding Soft acids are cations with a moderate positive charge (2+ or lower), Or cations with d electrons readily availbale for π-bonding ...
... or cations with d electrons not available for π-bonding Soft acids are cations with a moderate positive charge (2+ or lower), Or cations with d electrons readily availbale for π-bonding ...
the crystal and molecular structure of cyclopenta
... chloride, dicyclopentadienyltin(I1) does not show acceptor character, we suggest that the “empty” p orbital of the tin atom in dicyclopentadienyltin(I1) and in cyclopentadienyltin(I1) chloride is actually occupied as a result of the donation of a electron density from the cyclopentadienyl ring into ...
... chloride, dicyclopentadienyltin(I1) does not show acceptor character, we suggest that the “empty” p orbital of the tin atom in dicyclopentadienyltin(I1) and in cyclopentadienyltin(I1) chloride is actually occupied as a result of the donation of a electron density from the cyclopentadienyl ring into ...
Ruthenium(II/III) - Publications of the IAS Fellows
... higher energy bands in the UV region are of intra-ligand p–p* type or charge-transfer transitions involving energy levels which are higher in energy than the ligand lowest unoccupied molecular orbital (LUMO). It may be noted that for the Ru(bpy) 21 complex the ...
... higher energy bands in the UV region are of intra-ligand p–p* type or charge-transfer transitions involving energy levels which are higher in energy than the ligand lowest unoccupied molecular orbital (LUMO). It may be noted that for the Ru(bpy) 21 complex the ...
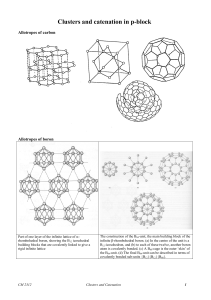
Clusters
... Molecules, ions or molecular fragments that possess the same number of valence and core electrons are said to be isoelectronic. However, this term is often used to refer to species that simply possess the same number of valence electrons. Example: BN is isoelectronic with CC The structure of layered ...
... Molecules, ions or molecular fragments that possess the same number of valence and core electrons are said to be isoelectronic. However, this term is often used to refer to species that simply possess the same number of valence electrons. Example: BN is isoelectronic with CC The structure of layered ...
Name: (1 of 2) Math Set # 13 Protons,
... now has a charge. For example, if a hydrogen atom has one proton (+) and one electron (-‐) the two charges cancel each other out. When the electron is lost the hydrogen atom is only a ...
... now has a charge. For example, if a hydrogen atom has one proton (+) and one electron (-‐) the two charges cancel each other out. When the electron is lost the hydrogen atom is only a ...
GCE Advanced Theoretical INORGANIC Chemistry Revision Notes
... the oxidation: Mg(s) ==> Mg2+(aq) + 2e- (ox. state increases by 2 units per Mg atom) the reduction: 2H+(aq) + 2e- ==> H2(aq) (ox. state decreases by 1 unit per H+ ion) o The oxidation state for magnesium is increased by two units per atom, is balanced by the oxidation state of two hydrogens (as ...
... the oxidation: Mg(s) ==> Mg2+(aq) + 2e- (ox. state increases by 2 units per Mg atom) the reduction: 2H+(aq) + 2e- ==> H2(aq) (ox. state decreases by 1 unit per H+ ion) o The oxidation state for magnesium is increased by two units per atom, is balanced by the oxidation state of two hydrogens (as ...
1. 1. Write down the electronic configuration of: 2. Why are Mn 2+
... covalent) are coloured both in the solid state and in aqueous solution in contrast to compounds of S and P block elements. This property is due to presence of unpaired electron(s) in the d-orbital and its transition within the d-orbtials. The energy of excitation corresponds to the frequency of ligh ...
... covalent) are coloured both in the solid state and in aqueous solution in contrast to compounds of S and P block elements. This property is due to presence of unpaired electron(s) in the d-orbital and its transition within the d-orbtials. The energy of excitation corresponds to the frequency of ligh ...
Fluorescence in Inorganic Analysis
... Diamagnetic metal ion complexes The formation of a fluorescent chelate by the combination of a diamagnetic metal ion and an organic ligand proves to be a sensitive and specific method for many metal ions, particularly those which are difficult to measure by atomic absorption spectroscopy. The basic ...
... Diamagnetic metal ion complexes The formation of a fluorescent chelate by the combination of a diamagnetic metal ion and an organic ligand proves to be a sensitive and specific method for many metal ions, particularly those which are difficult to measure by atomic absorption spectroscopy. The basic ...
Reactions in which some elements change their
... If the activation energy is high then it may need an extra "push" to get it going. - for example the reaction between chlorine and hydrogen needs a spark or ultraviolet light and then it is explosively fast. ...
... If the activation energy is high then it may need an extra "push" to get it going. - for example the reaction between chlorine and hydrogen needs a spark or ultraviolet light and then it is explosively fast. ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.