THE DETERMINATION OF TRANSITION METAL IONS IN
... over DTC's is the fact that metal-oxine complexes are more stable that metal-DTC complexes. Most of the metal-oxinate complexes are soluble in chloroform or other non-polar solvents. The reaction which occurs when an aqueous solution of a divalent metal ion, M ...
... over DTC's is the fact that metal-oxine complexes are more stable that metal-DTC complexes. Most of the metal-oxinate complexes are soluble in chloroform or other non-polar solvents. The reaction which occurs when an aqueous solution of a divalent metal ion, M ...
Room temperature ionic liquid as a novel medium for liquid/liquid
... Room temperature ionic liquids (RTILs) have been used as novel solvents to replace traditional volatile organic solvents in organic synthesis, solvent extraction, and electrochemistry. The hydrophobic character and water immiscibility of certain ionic liquids allow their use in solvent extraction of ...
... Room temperature ionic liquids (RTILs) have been used as novel solvents to replace traditional volatile organic solvents in organic synthesis, solvent extraction, and electrochemistry. The hydrophobic character and water immiscibility of certain ionic liquids allow their use in solvent extraction of ...
Syllabus of the International Chemistry Olympiad
... programs and need not to be mentioned in the preparatory problems. Level 2: These topics are included in a substantial number of secondary school programs and maybe used without exemplification in the preparatory problems. Level 3: These topics are not included in the majority of secondary school pr ...
... programs and need not to be mentioned in the preparatory problems. Level 2: These topics are included in a substantial number of secondary school programs and maybe used without exemplification in the preparatory problems. Level 3: These topics are not included in the majority of secondary school pr ...
Nomenclature Notes
... this system, organic ligands would receive substitutive names. Then, to be used in an additive context, organometallic compounds almost always require such a nomenclature mixture. The additive system may be used to name some compounds which would not usually be considered to be coordination complexe ...
... this system, organic ligands would receive substitutive names. Then, to be used in an additive context, organometallic compounds almost always require such a nomenclature mixture. The additive system may be used to name some compounds which would not usually be considered to be coordination complexe ...
Net Ionic Prep Session NMSI INSTRUCTOR
... weak Lewis base), the product is F3BNH3 (just smash everything together) since nitrogen donated its unshared electron pair to boron in an act of extreme generosity and formed a coordinate covalent bond. Lewis Acids Accept an electron pair. * The “little guys” in the IIA’s have solubility issues, wri ...
... weak Lewis base), the product is F3BNH3 (just smash everything together) since nitrogen donated its unshared electron pair to boron in an act of extreme generosity and formed a coordinate covalent bond. Lewis Acids Accept an electron pair. * The “little guys” in the IIA’s have solubility issues, wri ...
The synthesis and properties of solution processable red-emitting phosphorescent dendrimers Materials Chemistry
... from 3 occurs primarily or entirely from the localised MLCT states associated with the BTP ligand. The red shift in the PL spectrum of G1-BTP3Ir 9 relative to 3 is consistent with the longer conjugation length of the ligand chromophore. For both 3 and 9 the film PL spectra were similar to the solut ...
... from 3 occurs primarily or entirely from the localised MLCT states associated with the BTP ligand. The red shift in the PL spectrum of G1-BTP3Ir 9 relative to 3 is consistent with the longer conjugation length of the ligand chromophore. For both 3 and 9 the film PL spectra were similar to the solut ...
5.1-Organizing the elements - Environmental-Chemistry
... the right side of P.T. Nonmetals include some elements from gr. 13-16 and all elements of gr. 17 (halogens) and 18 (noble gases). • Outer most shell in noble gases is completely filled, so they are chemically inert with other elements, exist as single atom. They neither lose, nor gain electron since ...
... the right side of P.T. Nonmetals include some elements from gr. 13-16 and all elements of gr. 17 (halogens) and 18 (noble gases). • Outer most shell in noble gases is completely filled, so they are chemically inert with other elements, exist as single atom. They neither lose, nor gain electron since ...
A Carboxylate Oxygen of the Substrate Bridges
... which binds with higher affinity,2 metal 1, coordinates to the protein through the carboxylate side chains of Asp 246, Asp 320, and Glu 295 (Lebioda & Stec, 1989). In Mg2+enolase, three water molecules complete the octahedral coordination shell of metal 1 (Wedekind et al., 1995). The affinity of the ...
... which binds with higher affinity,2 metal 1, coordinates to the protein through the carboxylate side chains of Asp 246, Asp 320, and Glu 295 (Lebioda & Stec, 1989). In Mg2+enolase, three water molecules complete the octahedral coordination shell of metal 1 (Wedekind et al., 1995). The affinity of the ...
A.P. Chemistry Writing Chemical Reactions Generally students do
... SALTS : the salts which are soluble include all of the salts of lithium, sodium, potassium and ammonium cations and of nitrate and acetate anions. All chlorides are soluble except silver, lead and mercury(I) [AP H]. All sulfates are soluble except those of calcium, lead, barium, and strontium [C PBS ...
... SALTS : the salts which are soluble include all of the salts of lithium, sodium, potassium and ammonium cations and of nitrate and acetate anions. All chlorides are soluble except silver, lead and mercury(I) [AP H]. All sulfates are soluble except those of calcium, lead, barium, and strontium [C PBS ...
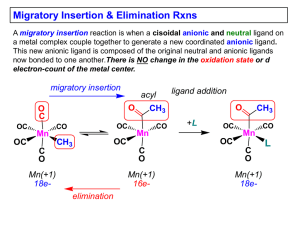
Slide 1
... General Features of Migratory Insertions: 1) No change in formal oxidation state (exception: alkylidenes) 2) The two groups that react must be cisoidal to one another 3) A vacant coordination site is generated by the migratory insertion. Therefore, a vacant site is required for the back elimination ...
... General Features of Migratory Insertions: 1) No change in formal oxidation state (exception: alkylidenes) 2) The two groups that react must be cisoidal to one another 3) A vacant coordination site is generated by the migratory insertion. Therefore, a vacant site is required for the back elimination ...
Unit5C - OCCC.edu
... • Electrons are not explicitly shown in chemical equations. • Oxidation Numbers are used to keep track of electrons gained and lost during redox reactions. ...
... • Electrons are not explicitly shown in chemical equations. • Oxidation Numbers are used to keep track of electrons gained and lost during redox reactions. ...
Electrochemical and Spectral Probes of Metal/Ligand Orbital Mixing in
... (via substituents) and monitored at the metal center (see footnote 18) presumably would measure mixing between all x* levels and the highest lying dx(Ru) orbital. This distinction might account for the slightly greater degree of mixing inferred from that experiment. Note, however, that the ability o ...
... (via substituents) and monitored at the metal center (see footnote 18) presumably would measure mixing between all x* levels and the highest lying dx(Ru) orbital. This distinction might account for the slightly greater degree of mixing inferred from that experiment. Note, however, that the ability o ...
Density Functional Theory Study of the Interaction of 2
... observe that the latest orbital 5s0 is free of electrons and it can accept two electrons, which can be provided by the free electronic doublet of deprotonated nitrogen atom of 2MBI molecule. This is in order to allow to Pd atom to have the stable configuration as [Kr]4d105s2. This is why after the o ...
... observe that the latest orbital 5s0 is free of electrons and it can accept two electrons, which can be provided by the free electronic doublet of deprotonated nitrogen atom of 2MBI molecule. This is in order to allow to Pd atom to have the stable configuration as [Kr]4d105s2. This is why after the o ...
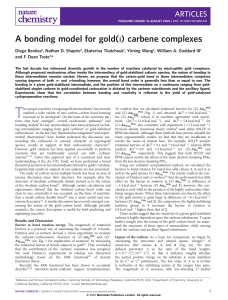
A Bonding Model for Gold(I) Carbene Complexes
... Impact of the ligand1,25. The computed structures of AuMeL, for various ligands (L) are shown in Fig. 3c. The L–Au2C1 bonding network can be partitioned into three components (Fig. 3d)26,27. Because there is only one vacant valence orbital on gold (6s), the ...
... Impact of the ligand1,25. The computed structures of AuMeL, for various ligands (L) are shown in Fig. 3c. The L–Au2C1 bonding network can be partitioned into three components (Fig. 3d)26,27. Because there is only one vacant valence orbital on gold (6s), the ...
PREPARATIONS AND APPLICATION OF METAL NANOPARTICLES
... is known as overlap of atomic orbitals to give nearly continuous electronic energy levels. The energy difference between the valence and conduction is called band gap (Eg). Metals that are electronically categorized as semiconductor have partially filled band (the valence band) separated from the (m ...
... is known as overlap of atomic orbitals to give nearly continuous electronic energy levels. The energy difference between the valence and conduction is called band gap (Eg). Metals that are electronically categorized as semiconductor have partially filled band (the valence band) separated from the (m ...
Chapter 1 Structure and Bonding
... Add OH-. CoS, ZnS, MnS, NiS, FeS, Cr(OH)3, and Al(OH)3 precipitate. ...
... Add OH-. CoS, ZnS, MnS, NiS, FeS, Cr(OH)3, and Al(OH)3 precipitate. ...
Lead(II) removal from aqueous solutions by solvent extraction with
... glass flask at 1:1 volume ratio of aqueous and organic phases. The chloroform phase of R(COOH)4 and aqueous phase containing metal nitrate were agitated by mechanical shaker with a constant stirring rate 600 rpm. The phases were separated after the mixture was settled for 12 h and the metal ion conc ...
... glass flask at 1:1 volume ratio of aqueous and organic phases. The chloroform phase of R(COOH)4 and aqueous phase containing metal nitrate were agitated by mechanical shaker with a constant stirring rate 600 rpm. The phases were separated after the mixture was settled for 12 h and the metal ion conc ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.