Advanced Higher Chemistry Resource Guide
... pair/bonding pair. These different strengths of electron pair repulsion account for slight deviations from expected bond angles in molecules such as NH3 and H2O. Transition metals The d-block transition metals are metals with an incomplete d subshell in at least one of their ions. The filling of the ...
... pair/bonding pair. These different strengths of electron pair repulsion account for slight deviations from expected bond angles in molecules such as NH3 and H2O. Transition metals The d-block transition metals are metals with an incomplete d subshell in at least one of their ions. The filling of the ...
coordination of some monodentate and hybrid multident ate
... A range of low-valent late transition-metal triarylphosphine complexes have been prepared and characterised by a combination of *H, [P and 19F (as appropriate) NMR and IR spectroscopies and mass spectrometry. Some of these complexes have been isolated as single crystals and characterised by X-ray di ...
... A range of low-valent late transition-metal triarylphosphine complexes have been prepared and characterised by a combination of *H, [P and 19F (as appropriate) NMR and IR spectroscopies and mass spectrometry. Some of these complexes have been isolated as single crystals and characterised by X-ray di ...
Module-2-s-and-d-elements - Львівський національний медичний
... view until the latter half of the 18th century. In 1781 the British chemist Henry Cavendish synthesized water by detonating a mixture of hydrogen and the air. However, the results of his experiments were not clearly interpreted until two years later, when the French chemist Antoine Laurent Lavoisie ...
... view until the latter half of the 18th century. In 1781 the British chemist Henry Cavendish synthesized water by detonating a mixture of hydrogen and the air. However, the results of his experiments were not clearly interpreted until two years later, when the French chemist Antoine Laurent Lavoisie ...
Chemistry 11 – Course Review
... Element “X” is actually the real element ________________________________. Regions in space occupied by electrons are called ___________________________ Write the ground state electron configurations (eg. 1s2 2s2 2p6) for the following atoms or ions. You may use the core notation. a) ...
... Element “X” is actually the real element ________________________________. Regions in space occupied by electrons are called ___________________________ Write the ground state electron configurations (eg. 1s2 2s2 2p6) for the following atoms or ions. You may use the core notation. a) ...
Chemical Bonding
... of them. For example, you know that iron is a solid at SATP (not a liquid or a gas), a metal (not a nonmetal), and an element (not a compound). Similarly, water is a liquid and a compound. In this section, we will look at ways of further classifying compounds. One category of compounds includes tabl ...
... of them. For example, you know that iron is a solid at SATP (not a liquid or a gas), a metal (not a nonmetal), and an element (not a compound). Similarly, water is a liquid and a compound. In this section, we will look at ways of further classifying compounds. One category of compounds includes tabl ...
November 2016 (v3) QP - Paper 4 CIE Chemistry A-level
... from ............................................................ to ............................................................ [1] ...
... from ............................................................ to ............................................................ [1] ...
November 2016 (v1) QP - Paper 4 CIE Chemistry A-level
... from ............................................................ to ............................................................ [1] ...
... from ............................................................ to ............................................................ [1] ...
Hydrides: Solid State Transition Metal Complexes
... hydrides, are not yet fully explored. The only other soluble Tmetal hydride complex known is [FeH6 ]4− .21 The solid-state reaction products are mostly polycrystalline and often colored. They usually contain impurity phases (nonreacted T and/or binary M metal hydrides), but rarely contain single cry ...
... hydrides, are not yet fully explored. The only other soluble Tmetal hydride complex known is [FeH6 ]4− .21 The solid-state reaction products are mostly polycrystalline and often colored. They usually contain impurity phases (nonreacted T and/or binary M metal hydrides), but rarely contain single cry ...
O - gearju.com
... rule. Count the valence electrons in HNO3 (in bonds and in lone pairs). The result is 24, the same as the total number of valence electrons on three O atoms (3 × 6 = 18), one N atom (5), and one H atom (1). ...
... rule. Count the valence electrons in HNO3 (in bonds and in lone pairs). The result is 24, the same as the total number of valence electrons on three O atoms (3 × 6 = 18), one N atom (5), and one H atom (1). ...
Chemistry 6/e Steven S. Zumdahl and Susan A. Zumdahl
... • More than one oxidation state is often found. • Cations are often complex ions – species where the transition metal ion is surrounded by a certain number of ligands (Lewis bases). ...
... • More than one oxidation state is often found. • Cations are often complex ions – species where the transition metal ion is surrounded by a certain number of ligands (Lewis bases). ...
Figure 1.01a: (a.)The surface of a single grain of table salt.
... Figure 21.17: (a) The trans isomer of Co(en)2Cl2+ and its mirror image are identical (superimposable). (b) The cis isomer of Co(en)2Cl2+ and its mirror image are not superimposable and are thus a pair of optical isomers. ...
... Figure 21.17: (a) The trans isomer of Co(en)2Cl2+ and its mirror image are identical (superimposable). (b) The cis isomer of Co(en)2Cl2+ and its mirror image are not superimposable and are thus a pair of optical isomers. ...
Document
... authors to be the best method to separate scandium from the other elements. Chitosan is a linear polysaccharide which is mainly obtained by alkaline deacetylation of chitin, a naturally abundant ...
... authors to be the best method to separate scandium from the other elements. Chitosan is a linear polysaccharide which is mainly obtained by alkaline deacetylation of chitin, a naturally abundant ...
Chemistry and Problems of Industrial Water Part One - Wiley-VCH
... and most of the remaining 3% (fresh water) is frozen at the North and South Poles. Only a very small volume of fresh water (∼ 0.02%) is available in rivers, streams, aquifers, and springs as drinking and process water. The water cycle (evaporation, condensation, precipitation, percolation, and trans ...
... and most of the remaining 3% (fresh water) is frozen at the North and South Poles. Only a very small volume of fresh water (∼ 0.02%) is available in rivers, streams, aquifers, and springs as drinking and process water. The water cycle (evaporation, condensation, precipitation, percolation, and trans ...
Electronegativity and Bond Type: Predicting
... Extension Using Transition Metals It would be satisfying to independently confirm that the unfunctionalized EN values provide a means of separating bonding types. To this end a new set of compounds of known bond type was determined using binary compounds that contain at least one transition metal or ...
... Extension Using Transition Metals It would be satisfying to independently confirm that the unfunctionalized EN values provide a means of separating bonding types. To this end a new set of compounds of known bond type was determined using binary compounds that contain at least one transition metal or ...
Metal Fluorides: Tools for Structural and Computational Analysis of
... in favor of concerted phosphoryl transfer reactions for phosphate monoesters and anhydrides. These have ‘‘in-line’’ geometry for OD–P–OA in the transition state (TS), with variable associative or dissociative character (Scheme 1b) [10–12]. Isotope labeling studies have contributed historically to st ...
... in favor of concerted phosphoryl transfer reactions for phosphate monoesters and anhydrides. These have ‘‘in-line’’ geometry for OD–P–OA in the transition state (TS), with variable associative or dissociative character (Scheme 1b) [10–12]. Isotope labeling studies have contributed historically to st ...
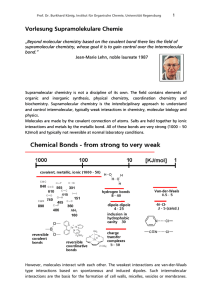
Vorlesung Supramolekulare Chemie
... Typical hydrogen bond donors: -OH, -NH, -(CO)NH, but also C-H is possible in special cases. Typical hydrogen bond acceptors: -OH, -O-, =N-, C=O. ...
... Typical hydrogen bond donors: -OH, -NH, -(CO)NH, but also C-H is possible in special cases. Typical hydrogen bond acceptors: -OH, -O-, =N-, C=O. ...
D AND F BLOCK ELEMENT
... (ii) Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high. (iii) Most of the complexes of transition metals are coloured. This is because ...
... (ii) Transition elements have high effective nuclear charge and a large number of valence electrons. Therefore, they form very strong metallic bonds. As a result, the enthalpy of atomization of transition metals is high. (iii) Most of the complexes of transition metals are coloured. This is because ...
CARBON-HYDROGEN-TRANSITION METAL BONDS
... bonds, especially those of saturated (s$) carbon centres, are normally regarded as being chemically inert. Generally, the C-H group is not thought of as a potential ligand which can have a structural role or play an energetically significant part in ground states or in reaction intermediates. In thi ...
... bonds, especially those of saturated (s$) carbon centres, are normally regarded as being chemically inert. Generally, the C-H group is not thought of as a potential ligand which can have a structural role or play an energetically significant part in ground states or in reaction intermediates. In thi ...
Dinesh-ohiostate06
... IR spectroscopy of M+(Acetone) (M=Mg+, Al+, Ca+) in the C=O stretch region reveals structures of these complexes. The C=O stretch shifts to lower frequencies due to M+ binding and can be explained electron density withdrawing mechanism of the bonding. The greatest shift is for the Al+ complex as the ...
... IR spectroscopy of M+(Acetone) (M=Mg+, Al+, Ca+) in the C=O stretch region reveals structures of these complexes. The C=O stretch shifts to lower frequencies due to M+ binding and can be explained electron density withdrawing mechanism of the bonding. The greatest shift is for the Al+ complex as the ...
Chapter 5 - DORAS
... Suzuki cross-coupling is one of the most widely exploited coupling reactions in the process of synthesizing heterogeneous compounds. Despite this method being the most direct route to the formation of a single asymmetric heterodinuclear complex, as shown in figure 5.1, the use of this approach did n ...
... Suzuki cross-coupling is one of the most widely exploited coupling reactions in the process of synthesizing heterogeneous compounds. Despite this method being the most direct route to the formation of a single asymmetric heterodinuclear complex, as shown in figure 5.1, the use of this approach did n ...
Fundamentals of Chemistry
... atom is an extremely small electrically-neutral particle. It is the smallest unit involved in the chemical change of matter. Atoms can be treated as distinct particles because they behave as such chemically, but atoms themselves are composed of even smaller subparts. Understanding these atomic subpa ...
... atom is an extremely small electrically-neutral particle. It is the smallest unit involved in the chemical change of matter. Atoms can be treated as distinct particles because they behave as such chemically, but atoms themselves are composed of even smaller subparts. Understanding these atomic subpa ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.