1 CHAPTER ONE Palladium in Organic Synthesis 1.1 Background
... However, one of the main complications that has been problematic in the development of Pd(II) methodology is the difficulty of reoxidizing Pd(0) → Pd(II). Completion of the catalytic cycle to regenerate Pd(II) requires the presence of a stoichiometric oxidant, such as CuCl2, Cu(OAc)2, benzoquinone, ...
... However, one of the main complications that has been problematic in the development of Pd(II) methodology is the difficulty of reoxidizing Pd(0) → Pd(II). Completion of the catalytic cycle to regenerate Pd(II) requires the presence of a stoichiometric oxidant, such as CuCl2, Cu(OAc)2, benzoquinone, ...
Sample Chapter - Chapter 4
... attraction between them. To see how it does this, let’s examine the water molecule closely. Water’s power as an ionizing solvent results from two features of the water molecule: the distribution of its bonding electrons and its overall shape. Recall from Section 2.7 that the electrons in a covalent ...
... attraction between them. To see how it does this, let’s examine the water molecule closely. Water’s power as an ionizing solvent results from two features of the water molecule: the distribution of its bonding electrons and its overall shape. Recall from Section 2.7 that the electrons in a covalent ...
IE in C-H activation - The School of Life Sciences at Sussex
... Over the past 30 years or so, a number of examples of carbon-hydrogen bond activation by transition metals have appeared in the literature.1 This work took on greater significance in 1982, when Bergman reported a reaction in which a “simple” oxidative addition of cyclohexane to a photochemically gen ...
... Over the past 30 years or so, a number of examples of carbon-hydrogen bond activation by transition metals have appeared in the literature.1 This work took on greater significance in 1982, when Bergman reported a reaction in which a “simple” oxidative addition of cyclohexane to a photochemically gen ...
Amines
... Aliphatic amines are stronger bases (lower pKb) than ammonia. This is because alkyl groups repel electrons, leading to an increase in negative charge around the nitrogen so that it more readily attracts and accepts an H+ ion. This means, 2° amines are more basic than 1° amines, and 3° amines are mor ...
... Aliphatic amines are stronger bases (lower pKb) than ammonia. This is because alkyl groups repel electrons, leading to an increase in negative charge around the nitrogen so that it more readily attracts and accepts an H+ ion. This means, 2° amines are more basic than 1° amines, and 3° amines are mor ...
Chemistry
... smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known ...
... smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, the chemist John Dalton, revived the term when he suggested that each element was made up of unique atoms and the atoms of an element are all the same. At that time, there were about 35 known ...
Manganese-Substituted Myoglobin: Characterization and Reactivity
... reported for decades, but investigations into the reactivity of transition metal-substituted myoglobin have received little attention. Transition metal complexes display varying reactivities with different transition metals even when the same types of ligands ligate them [26,27]. Therefore, it is re ...
... reported for decades, but investigations into the reactivity of transition metal-substituted myoglobin have received little attention. Transition metal complexes display varying reactivities with different transition metals even when the same types of ligands ligate them [26,27]. Therefore, it is re ...
Chemistry - University of Mysore
... of s, p and d orbitals. Effective nuclear charge, screening effect-based on Slater’s rules (problems to be worked out). General energy level diagram of multi electron atom (up to n=4). Pauli’s exclusion principle, Hund’s rule, (n+1) rule, Aufbau principle. Electronic configuration of elements (up to ...
... of s, p and d orbitals. Effective nuclear charge, screening effect-based on Slater’s rules (problems to be worked out). General energy level diagram of multi electron atom (up to n=4). Pauli’s exclusion principle, Hund’s rule, (n+1) rule, Aufbau principle. Electronic configuration of elements (up to ...
Electronic Structure of Cobalt Nanocrystals Suspended in Liquid
... charge transfer (MLCT) effects. The calculations describe the ground state as [3d7 + 3d6L] with the MLCT energy ∆π of -3.0 eV. This yields a ground state that has 38% 3d7 and 62% 3d6L, where it is noticed that the main L3 edge still looks similar to that of the 3d7 ground state found in CoO. The mai ...
... charge transfer (MLCT) effects. The calculations describe the ground state as [3d7 + 3d6L] with the MLCT energy ∆π of -3.0 eV. This yields a ground state that has 38% 3d7 and 62% 3d6L, where it is noticed that the main L3 edge still looks similar to that of the 3d7 ground state found in CoO. The mai ...
Chapter Two - Blackboard
... unit includes a fixed number of water molecules associated with cations and anions. • To name a hydrate, the compound name is followed by “___hydrate” where the blank is a prefix to indicate the number of water molecules. • The number of water molecules associated with each formula unit is written a ...
... unit includes a fixed number of water molecules associated with cations and anions. • To name a hydrate, the compound name is followed by “___hydrate” where the blank is a prefix to indicate the number of water molecules. • The number of water molecules associated with each formula unit is written a ...
SOLID STATE KEY CONCEPTS As we know that matter exists in different physical states under different conditions
... 14. The packing efficiency in bcc =2*4/3 *πr3/64*33/2 r3 *100 =68 15. The packing efficiency in hcp =74 16. Packing efficiency in bcc arrangement in 68% and simple cubic unit cell is 52.4% 17. Unoccupied spaces in solids are called interstitial voids or interstitial sites. 18. Two important in ...
... 14. The packing efficiency in bcc =2*4/3 *πr3/64*33/2 r3 *100 =68 15. The packing efficiency in hcp =74 16. Packing efficiency in bcc arrangement in 68% and simple cubic unit cell is 52.4% 17. Unoccupied spaces in solids are called interstitial voids or interstitial sites. 18. Two important in ...
PDF (Chapter 4)
... b. Cooperative Dioxygen Binding Many dioxygen-binding proteins are not independent monomers, with only one dioxygen-binding site, but oligomeric species with the protein comprising two or more similar subunits. The subunits may be held together by van der Waals' forces or by stronger interactions, s ...
... b. Cooperative Dioxygen Binding Many dioxygen-binding proteins are not independent monomers, with only one dioxygen-binding site, but oligomeric species with the protein comprising two or more similar subunits. The subunits may be held together by van der Waals' forces or by stronger interactions, s ...
inorganic chemistry
... electronegative due to their high effective nuclear charge. They can gain an electron by reacting with atoms of other elements. Fluorine is one of the most reactive elements in existence, attacking otherwise inert materials such as glass, and forming compounds with the heavier noble gases. It is a c ...
... electronegative due to their high effective nuclear charge. They can gain an electron by reacting with atoms of other elements. Fluorine is one of the most reactive elements in existence, attacking otherwise inert materials such as glass, and forming compounds with the heavier noble gases. It is a c ...
Linkage Isomerization in Heme-NOx Compounds: Understanding
... systems. We focused initially on the known {FeNO}7 fivecoordinate (por)Fe(NO) class of compounds for proof of concept because (i) many of these were known to be thermally stable in the ground state and (ii) these compounds could be obtained in elementally pure form.1 Photolysis of the (TTP)Fe(NO) co ...
... systems. We focused initially on the known {FeNO}7 fivecoordinate (por)Fe(NO) class of compounds for proof of concept because (i) many of these were known to be thermally stable in the ground state and (ii) these compounds could be obtained in elementally pure form.1 Photolysis of the (TTP)Fe(NO) co ...
crystals
... usually described as consisting of hexagonal closest packings of spheres of As and I, respectively, with all and half of the octahedral interstices filled with Ni and Cd atoms, respectively. Thus, the hexagonal unit cell with the parameters a0 and c0 contains two and one formula units, respectively. ...
... usually described as consisting of hexagonal closest packings of spheres of As and I, respectively, with all and half of the octahedral interstices filled with Ni and Cd atoms, respectively. Thus, the hexagonal unit cell with the parameters a0 and c0 contains two and one formula units, respectively. ...
Vibrational spectroscopy of bare and solvated ionic complexes of
... structures of biologically relevant molecules. Particularly in combination with modern electronic structure calculations, such as density functional theory (DFT), IR spectroscopy has become a powerful structural tool in MS research that is now being applied in a growing number of labs around the wor ...
... structures of biologically relevant molecules. Particularly in combination with modern electronic structure calculations, such as density functional theory (DFT), IR spectroscopy has become a powerful structural tool in MS research that is now being applied in a growing number of labs around the wor ...
2.6 M - Thierry Karsenti
... If you get 6 items or more correct you can consider that you are doing fine, but if you get less than 4 items correct then you have to work very hard to pass the course. ...
... If you get 6 items or more correct you can consider that you are doing fine, but if you get less than 4 items correct then you have to work very hard to pass the course. ...
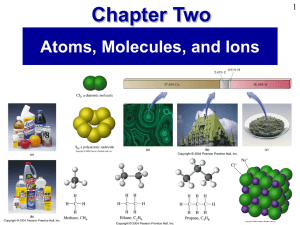
Prentice Hall Ch 02 Atoms Molecules Ions
... • A molecule is a group of two or more atoms held together by covalent bonds. • A molecular formula gives the number of each kind of atom in a molecule. • An empirical formula simply gives the (whole number) ratio of atoms of elements in a compound. Compound ...
... • A molecule is a group of two or more atoms held together by covalent bonds. • A molecular formula gives the number of each kind of atom in a molecule. • An empirical formula simply gives the (whole number) ratio of atoms of elements in a compound. Compound ...
STUDY MATERIAL 2015-16 CHEMISTRY CLASS XI
... such as minerals. For example, common salt, marble and limestone. Organic compounds are those, which occur in living sources such as plants and animals. They all contain carbon. Common organic compounds are oils, wax, fats etc. Mixtures A mixture is a combination of two or more elements or compounds ...
... such as minerals. For example, common salt, marble and limestone. Organic compounds are those, which occur in living sources such as plants and animals. They all contain carbon. Common organic compounds are oils, wax, fats etc. Mixtures A mixture is a combination of two or more elements or compounds ...
towards the synthesis of functionalised macrocyclic receptors
... Supramolecular chemistry impinges on numerous, cognate, areas of research although those which are most germane to this discussion are host-guest chemistry and self-assembly. ...
... Supramolecular chemistry impinges on numerous, cognate, areas of research although those which are most germane to this discussion are host-guest chemistry and self-assembly. ...
BASIC CONCEPTS OF CHEMISTRY
... HCl + NaOH = NaCl + H2O (for monobasic acids only one reaction of neutralisation is possible). For the multibasic acids full and incomplete neutralisation is possible: H2SО4 + 2КОН = К2SО4 + 2Н2О (full neutralisation) H2SО4 + КОН = КНSО4 + Н2О (incomplete neutralization) ...
... HCl + NaOH = NaCl + H2O (for monobasic acids only one reaction of neutralisation is possible). For the multibasic acids full and incomplete neutralisation is possible: H2SО4 + 2КОН = К2SО4 + 2Н2О (full neutralisation) H2SО4 + КОН = КНSО4 + Н2О (incomplete neutralization) ...
Design and Synthesis of a Thermally Stable Organic Electride
... serve to isolate the cations from electrons trapped in intermolecular cavities and channels.1-7 Electrides provide unique examples of low-density electron gases confined to cavities and channels of known geometry, which makes them related to both salts and plasmas. Therefore, they are of interest in ...
... serve to isolate the cations from electrons trapped in intermolecular cavities and channels.1-7 Electrides provide unique examples of low-density electron gases confined to cavities and channels of known geometry, which makes them related to both salts and plasmas. Therefore, they are of interest in ...
pdf version of ibook - mvhs
... (charge) such as Al3+ and Na+ (generally group 1, 2 or 13 metal cations) or show variable valency (generally transition metal cations). The anion can be monoatomic such as Cl-, S2-, P3- or Polyatomic such as NO3- or SO42-. The rules for naming ionic compounds with single valency cation, variable val ...
... (charge) such as Al3+ and Na+ (generally group 1, 2 or 13 metal cations) or show variable valency (generally transition metal cations). The anion can be monoatomic such as Cl-, S2-, P3- or Polyatomic such as NO3- or SO42-. The rules for naming ionic compounds with single valency cation, variable val ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.