SYNTHESIS, PHYSICO-CHEMICAL STUDIES AND ANTIMICROBIAL EVALUATION OF NOVEL 2- H COMPLEXES Research Article
... TGA of [M(BTa)2Cl2] (M = Cu(II), Ni(II) and Co(II)) All complexes show isothermic degradation with two or more steps of decomposition. The copper(II) complex have 27% weight degradation at 130-320 °C may be due to loss of chloride ions and methoxy groups, second decomposition start at 320 °C and fin ...
... TGA of [M(BTa)2Cl2] (M = Cu(II), Ni(II) and Co(II)) All complexes show isothermic degradation with two or more steps of decomposition. The copper(II) complex have 27% weight degradation at 130-320 °C may be due to loss of chloride ions and methoxy groups, second decomposition start at 320 °C and fin ...
Chapter 19: Molecules and Compounds
... Ratio of atoms bonded together in a compound, i.e. X:Y General Form: AxBy where x and y are called subscripts. ...
... Ratio of atoms bonded together in a compound, i.e. X:Y General Form: AxBy where x and y are called subscripts. ...
Atom (A) or Ion
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
Atom (A) or Ion (I)
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
... 82. What is pH and how is it measured? 83. What factors affect solubility? 84. What is molarity? 85. If I have 2.5 mol of calcium carbonate in .3 L of solution, what is the molarity? 86. If I have 700 mL of a 5 M NaOH solution, how many grams of NaOH were used to make the solution? 87. What are coll ...
Coordination Compounds Coordination Compounds
... compounds are called coordination compounds. The chemistry of coordination compounds is an important and challenging area of modern inorganic chemistry. New concepts of chemical bonding and molecular structure have provided insights into the functioning of vital components of biological systems. Chl ...
... compounds are called coordination compounds. The chemistry of coordination compounds is an important and challenging area of modern inorganic chemistry. New concepts of chemical bonding and molecular structure have provided insights into the functioning of vital components of biological systems. Chl ...
SEPARATION OF MAGNESIUM
... Several series of buffered authentic solutions with pH ranging from about 3 to 8 were prepared for each of Mg(II) , Mn(II) and Zn (II) ions . Within each series the metal ion concentration was between 1 × 10-2 – 1 × 10-4 M.To ( 5mL ) of the buffered metallic ion solution an equal volume of dioxin to ...
... Several series of buffered authentic solutions with pH ranging from about 3 to 8 were prepared for each of Mg(II) , Mn(II) and Zn (II) ions . Within each series the metal ion concentration was between 1 × 10-2 – 1 × 10-4 M.To ( 5mL ) of the buffered metallic ion solution an equal volume of dioxin to ...
Chapter 4 REVIEW
... 21. Ionic compounds and metals have different physical properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large differe ...
... 21. Ionic compounds and metals have different physical properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large differe ...
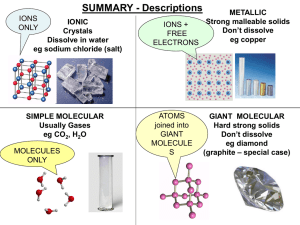
smart_materials_1 - Aldercar High School
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
... Regular structure, layers slide CONDUCT: YES (very well) Free electrons between ions ...
Qualitative and Quantitative Analysis of Metal Complexes of Schiff
... anions from the neighbouring molecules. A similar situation was also found in the crystal structure of the [Ti III(H 2L)I 2]I.0.5I 2 (H 2L = bis(hydrazone 2,6 diacetylpyridine)) complex. Finally, the obtained results indicate the differences in the possibility of the formation of trihalogenopyridine ...
... anions from the neighbouring molecules. A similar situation was also found in the crystal structure of the [Ti III(H 2L)I 2]I.0.5I 2 (H 2L = bis(hydrazone 2,6 diacetylpyridine)) complex. Finally, the obtained results indicate the differences in the possibility of the formation of trihalogenopyridine ...
Chapter
... • coordination number range from 2 to 12, with the most common being 6 and 4 CoCl36H2O = [Co(H2O)6]Cl3 Tro, Chemistry: A Molecular Approach ...
... • coordination number range from 2 to 12, with the most common being 6 and 4 CoCl36H2O = [Co(H2O)6]Cl3 Tro, Chemistry: A Molecular Approach ...
Three 1D cyanide-bridged M(Ni, Pd, Pt)
... diamine are good auxiliary ligands for assembling cyanide-bridged magnetic complexes by incorporating some paramagnetic metal ions such as Mn(II), Fe(II) and Co(II), etc.24–31 With comparison to the above two macrocyclic ligands, the semi-closed pentadentate macrocycles ligand used here (Scheme 1) m ...
... diamine are good auxiliary ligands for assembling cyanide-bridged magnetic complexes by incorporating some paramagnetic metal ions such as Mn(II), Fe(II) and Co(II), etc.24–31 With comparison to the above two macrocyclic ligands, the semi-closed pentadentate macrocycles ligand used here (Scheme 1) m ...
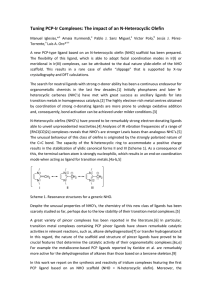
Lecture 6 - TCD Chemistry
... Orbitals with π character can interact with the t2g d orbitals – Must be correct symmetry (t2g) 3 arrangements possible ...
... Orbitals with π character can interact with the t2g d orbitals – Must be correct symmetry (t2g) 3 arrangements possible ...
Complexometric Reactions and Titrations
... an electron rich, and thus, electron donating species. A metal will thus accept electrons from a ligand where coordination bonds are formed. Electrons forming coordination bonds come solely from ligands. ...
... an electron rich, and thus, electron donating species. A metal will thus accept electrons from a ligand where coordination bonds are formed. Electrons forming coordination bonds come solely from ligands. ...
MnAcacfinal - Chemistry at Winthrop University
... 3. Ligand Identity D-orbital Splitting Octahedral (Do) ...
... 3. Ligand Identity D-orbital Splitting Octahedral (Do) ...
Document
... The number of atoms of the element in the compound is represented by its subscript. NOTE: ...
... The number of atoms of the element in the compound is represented by its subscript. NOTE: ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.