Microporous Porphyrin and Metalloporphyrin Materials
... chloro- and bromo- derivatives. However, the nonbonding F2F contact distances have increased to 4.1 and 4.2 A> , respectively, characteristic of a repulsive interaction rather than attractive force as observed in other halogenated porphyrin materials (7). Tightly packed herringbone-like layers chara ...
... chloro- and bromo- derivatives. However, the nonbonding F2F contact distances have increased to 4.1 and 4.2 A> , respectively, characteristic of a repulsive interaction rather than attractive force as observed in other halogenated porphyrin materials (7). Tightly packed herringbone-like layers chara ...
Characterization of Low Energy Charge Transfer Transitions in
... the HOMO being largely ruthenium-centered and the LUMO largely tpy-centered. The most intense contribution to a lowest energy MLCT absorption envelope (band III) of these complexes corresponds to the convolution of several orbitally different components, and its absorption maximum has an energy that ...
... the HOMO being largely ruthenium-centered and the LUMO largely tpy-centered. The most intense contribution to a lowest energy MLCT absorption envelope (band III) of these complexes corresponds to the convolution of several orbitally different components, and its absorption maximum has an energy that ...
Word 97, TIFF, Unbekannt, Corel Draw, Windows
... To clarify this apparent contradiction, the electrical properties of two-, quasi-two and three-dimensional systems were investigated. The measurements indicate that Simon’s model is valid for physically cross-linked systems only. For covalently (chemically) crosslinked nanoparticle systems the activ ...
... To clarify this apparent contradiction, the electrical properties of two-, quasi-two and three-dimensional systems were investigated. The measurements indicate that Simon’s model is valid for physically cross-linked systems only. For covalently (chemically) crosslinked nanoparticle systems the activ ...
Two-electron Quenching of Dinuclear Ruthenium(II)
... Atom color code: carbon, grey; nitrogen, blue. Right: The structure for phen…………100 ...
... Atom color code: carbon, grey; nitrogen, blue. Right: The structure for phen…………100 ...
noble gases
... 35. Cuprous ion is colourless while cupric ion is coloured because a. Cu+ ion has a complete d-orbital and Cu2+ has incomplete d-orbital b. Both have unpaired p electrons in d-orbital c. Cu+ has incomplete d-orbital and Cu2+ ion has complete dorbitals d. Both have half filled orbitals Ans : a Cu+ i ...
... 35. Cuprous ion is colourless while cupric ion is coloured because a. Cu+ ion has a complete d-orbital and Cu2+ has incomplete d-orbital b. Both have unpaired p electrons in d-orbital c. Cu+ has incomplete d-orbital and Cu2+ ion has complete dorbitals d. Both have half filled orbitals Ans : a Cu+ i ...
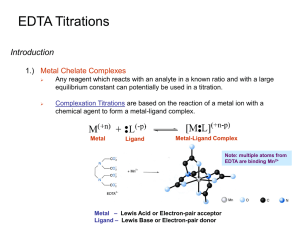
Chapter 12: EDTA Titrations - UNL
... Usually chelating agents with more than one electron pair to donate will form stronger complexes with metal ions than chelating agents with only one electron pair. ...
... Usually chelating agents with more than one electron pair to donate will form stronger complexes with metal ions than chelating agents with only one electron pair. ...
Transformation of a Metal−Organic Framework from the NbO to PtS
... Here again both Co(II) centers have distorted octahedral geometries and are bridged by three carboxylates: two bismonodentate and one chelating µ2-O bridging tridentate (Figure 2b). One Co(II) center has a single water and two terminal DMF molecules while the other has two chelating bidentate carbox ...
... Here again both Co(II) centers have distorted octahedral geometries and are bridged by three carboxylates: two bismonodentate and one chelating µ2-O bridging tridentate (Figure 2b). One Co(II) center has a single water and two terminal DMF molecules while the other has two chelating bidentate carbox ...
CHM203 - National Open University of Nigeria
... strength of a hydrogen bond ranges from 10 to 40 kJ mol-1. Hydrogen bonding has an important influence on physical properties such as melting point, boiling point and solubility of substances. This will be illustrated using examples in the following subsections. The dipole-dipole, induced dipole-ind ...
... strength of a hydrogen bond ranges from 10 to 40 kJ mol-1. Hydrogen bonding has an important influence on physical properties such as melting point, boiling point and solubility of substances. This will be illustrated using examples in the following subsections. The dipole-dipole, induced dipole-ind ...
1 - DORAS
... Firstly I have to thank Han. At all times you were supportive, helpful and ready to offer a joke whenever things were getting tough. I a m grateful to have worked with you for the past four years and you were an excellent mentor. Plus, no longer will I think of the Dutch as just those people w h o a ...
... Firstly I have to thank Han. At all times you were supportive, helpful and ready to offer a joke whenever things were getting tough. I a m grateful to have worked with you for the past four years and you were an excellent mentor. Plus, no longer will I think of the Dutch as just those people w h o a ...
BRAZING AND BRAZE WELDING Fluxes
... 1. Sufficient fluidity so the metal will flow evenly by capillary attraction. 2. Good melting action to form a sound metallurgical bond. 3. Melting point consistent with the type of metal to be joined. Brazing filler metals fall into seven groups : silver, aluminium-silicon, copper-phosphorus, gold, ...
... 1. Sufficient fluidity so the metal will flow evenly by capillary attraction. 2. Good melting action to form a sound metallurgical bond. 3. Melting point consistent with the type of metal to be joined. Brazing filler metals fall into seven groups : silver, aluminium-silicon, copper-phosphorus, gold, ...
Chemistry
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
pdf
... strains containing NiRK and NiRS. The objective was to probe the mechanism of Cu acquisition using ligands with selective affinities for Cu(I) and Cu(II) comparable in strength to natural ligands. Very strong ligands enable us to study the relationship between Cu limitation and free Cu2+ concentrati ...
... strains containing NiRK and NiRS. The objective was to probe the mechanism of Cu acquisition using ligands with selective affinities for Cu(I) and Cu(II) comparable in strength to natural ligands. Very strong ligands enable us to study the relationship between Cu limitation and free Cu2+ concentrati ...
Grignard knowledge: Alkyl coupling chemistry with inexpensive
... species to a lesser positive charge, a neutral state, or even negative charge. Some catalytic cycles depend on an in situ reduction of the transition metal in the process. Grignard reagents can transfer the R anion group of RMgX from the magnesium to catalyst M to afford a transient R-M(-) species. ...
... species to a lesser positive charge, a neutral state, or even negative charge. Some catalytic cycles depend on an in situ reduction of the transition metal in the process. Grignard reagents can transfer the R anion group of RMgX from the magnesium to catalyst M to afford a transient R-M(-) species. ...
Nitrene Transfer Reactions Mediated by Transition
... Nitrene Transfer Reactions Mediated by Transition Metal Scorpionate Complexes Director of Dissertation: Michael P. Jensen Transition metal catalyzed C=C bond aziridination and C-H bond amination reactions are powerful synthetic methods for forming C-N bonds directly from unfunctionalized hydrocarbon ...
... Nitrene Transfer Reactions Mediated by Transition Metal Scorpionate Complexes Director of Dissertation: Michael P. Jensen Transition metal catalyzed C=C bond aziridination and C-H bond amination reactions are powerful synthetic methods for forming C-N bonds directly from unfunctionalized hydrocarbon ...
No Slide Title
... The larger the stability constant, the further the reaction lies to the right Complex ions with large stability constants are more stable Stability constants are often given as pKstab Complex ions with smaller pKstab values are more stable ...
... The larger the stability constant, the further the reaction lies to the right Complex ions with large stability constants are more stable Stability constants are often given as pKstab Complex ions with smaller pKstab values are more stable ...
06_chapter 1
... separation. However, in most cases the heterogeneous catalysts give low ee. Polycrystalline nickel surface gives racemic products. It can be made chirally selective by suitable modification with chirally active modifiers like naturally occurring cinchona alkaloids and tartaric acid. The most reporte ...
... separation. However, in most cases the heterogeneous catalysts give low ee. Polycrystalline nickel surface gives racemic products. It can be made chirally selective by suitable modification with chirally active modifiers like naturally occurring cinchona alkaloids and tartaric acid. The most reporte ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.