Oxidative Addition - vrg
... H2 is by far the most important for catalytic applications, followed by Si-H bonds, B-H, N-H, and S-H bonds. C-H bond activation and functionalization is very important, but still not practical. ...
... H2 is by far the most important for catalytic applications, followed by Si-H bonds, B-H, N-H, and S-H bonds. C-H bond activation and functionalization is very important, but still not practical. ...
Inorganic Chemistry 412 / 512
... Potassium hydrogen phosphite (K2HPO3) is not stable in aqueous solution. Write a balanced redox reaction that occurs when it is added to aqueous base. [9 pts] HPO32- (aq) + OH- (l) ...
... Potassium hydrogen phosphite (K2HPO3) is not stable in aqueous solution. Write a balanced redox reaction that occurs when it is added to aqueous base. [9 pts] HPO32- (aq) + OH- (l) ...
TRANSITION METAL COMPLEXES AS
... • Metal = you must first determine the formal oxidation state of the metal. The number of electrons is the row number minus the charge on the metal. The formal oxidation state is simply the charge on the complex minus the charges of the ligands. • Ligands = in general L and X are both 2 electrons do ...
... • Metal = you must first determine the formal oxidation state of the metal. The number of electrons is the row number minus the charge on the metal. The formal oxidation state is simply the charge on the complex minus the charges of the ligands. • Ligands = in general L and X are both 2 electrons do ...
Organic Chemistry
... 1. Only electrons move! Nuclei and the sigma- (single bond-) framework are unchanged (Resonance occurs in the pi-system: conjugated lone pairs and pi-bonds). 2. Every resonance structure must be a valid Lewis structure. 3. Keep track of lone pairs and formal charges. 4. Use arrow-pushing formalism t ...
... 1. Only electrons move! Nuclei and the sigma- (single bond-) framework are unchanged (Resonance occurs in the pi-system: conjugated lone pairs and pi-bonds). 2. Every resonance structure must be a valid Lewis structure. 3. Keep track of lone pairs and formal charges. 4. Use arrow-pushing formalism t ...
25 Elements
... magnesium for light-weight alloys, and is also used in batteries, some greases, some glasses, and in medicine. ...
... magnesium for light-weight alloys, and is also used in batteries, some greases, some glasses, and in medicine. ...
Figure 2: Alternative Periodic Table
... e) Cl- or K+ 103) Compare the elements Li, K, C, N a) Which has the largest atomic radius? K b) Place the elements in order of increasing ionization energy. K < Li < C < N 109) Which group of the periodic table has elements with high first ionization potentials and very negative electron affinities? ...
... e) Cl- or K+ 103) Compare the elements Li, K, C, N a) Which has the largest atomic radius? K b) Place the elements in order of increasing ionization energy. K < Li < C < N 109) Which group of the periodic table has elements with high first ionization potentials and very negative electron affinities? ...
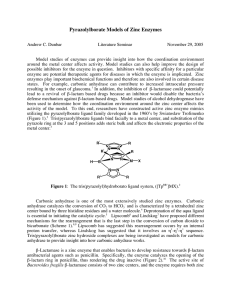
Pyrazolylborate Models of Zinc Enzymes
... Model studies of enzymes can provide insight into how the coordination environment around the metal center affects activity. Model studies can also help improve the design of possible inhibitors for the enzyme in question. Inhibitors with specific affinity for a particular enzyme are potential thera ...
... Model studies of enzymes can provide insight into how the coordination environment around the metal center affects activity. Model studies can also help improve the design of possible inhibitors for the enzyme in question. Inhibitors with specific affinity for a particular enzyme are potential thera ...
Dark-Field Oxidative Addition-Based Chemosensing: New Bis-cyclometalated Pt(II) Complexes and Phosphorescent
... in their phosphorescence energy. The rigidochromic effect on the emission of these complexes is a small value of 9 ( 3 nm upon freezing the sample in a 2-methyltetrahydrofuran glass. The rigid glass does not allow reorganization of solvent dipoles upon generation of excited states and give strongly ...
... in their phosphorescence energy. The rigidochromic effect on the emission of these complexes is a small value of 9 ( 3 nm upon freezing the sample in a 2-methyltetrahydrofuran glass. The rigid glass does not allow reorganization of solvent dipoles upon generation of excited states and give strongly ...
Coinage Metal−Ethylene Complexes Supported
... asymmetry.10 Alvarez’s group has published an interesting computational study of the bonding in group 11 complexes, focusing on preference in coordination mode.11 In a previous experiment-theory analysis of the coinage metal-tris(ethylene) cations, the metal-ligand bonding in these complexes was des ...
... asymmetry.10 Alvarez’s group has published an interesting computational study of the bonding in group 11 complexes, focusing on preference in coordination mode.11 In a previous experiment-theory analysis of the coinage metal-tris(ethylene) cations, the metal-ligand bonding in these complexes was des ...
Incorporation of Cyano Transition Metal Complexes in KCl Crystals
... results probing the incorporation of three additives, hexacyanoferrate(ii), hexacyanocobaltate(iii), and hexacyanoferrate(iii), into potassium chloride. The work to date in the literature has probed the preferred environments of the transition metal complexes in the crystal lattice, rather than focu ...
... results probing the incorporation of three additives, hexacyanoferrate(ii), hexacyanocobaltate(iii), and hexacyanoferrate(iii), into potassium chloride. The work to date in the literature has probed the preferred environments of the transition metal complexes in the crystal lattice, rather than focu ...
pblock - Chemistry Courses
... 2nd period: Only s and p orbitals are possible with n = 2 Therefore, the maximum number of bonds is 4 (single and/or double bonds) Examples: CH4, NF4+, BH43rd (and higher periods): can use d-orbitals to make bonds E.g. ...
... 2nd period: Only s and p orbitals are possible with n = 2 Therefore, the maximum number of bonds is 4 (single and/or double bonds) Examples: CH4, NF4+, BH43rd (and higher periods): can use d-orbitals to make bonds E.g. ...
in-situ by 1,2,4-triazole schiff bases and different metal salts A. Mouadili
... Ligands : L1-L11 Metals : Cu(CH3 CO2)2; Cu(NO3)2; ErCl3; NiCl2; CoCl2; ZnCl2. ...
... Ligands : L1-L11 Metals : Cu(CH3 CO2)2; Cu(NO3)2; ErCl3; NiCl2; CoCl2; ZnCl2. ...
Applied NanoWorks Announces New Water Soluble Titanate
... The company has tested the new molecule for several industry applications. As a traditional catalyst the water soluble titanyl improves Lewis Acid catalytic Diels-Alder reactions by 35%. Also, as an adhesion promoter it improved the tensile strength of carbon fiber epoxy bonding by 27%. Nanoparticle ...
... The company has tested the new molecule for several industry applications. As a traditional catalyst the water soluble titanyl improves Lewis Acid catalytic Diels-Alder reactions by 35%. Also, as an adhesion promoter it improved the tensile strength of carbon fiber epoxy bonding by 27%. Nanoparticle ...
5.New functional coordination compounds
... d-orbit energy level splitting (1) spherical symmetry field Due to the negative charge of ligands distributed spherically, no matter where d-orbit is, the repulsive interaction of the negative charge is the same, although of the d-orbital energy increases, still keep quintet degenerate d-orbitals. ...
... d-orbit energy level splitting (1) spherical symmetry field Due to the negative charge of ligands distributed spherically, no matter where d-orbit is, the repulsive interaction of the negative charge is the same, although of the d-orbital energy increases, still keep quintet degenerate d-orbitals. ...
Precipitation titration
... Indicators for EDTA titration : Many indicators are used for metal ion titration with EDTA . These indicators are organic dyes which form colored chelates with metal ions in range that is unique to the cation and dye . The colored complexes being visible to eye in range of 10-6M to 10-7M . Erochrome ...
... Indicators for EDTA titration : Many indicators are used for metal ion titration with EDTA . These indicators are organic dyes which form colored chelates with metal ions in range that is unique to the cation and dye . The colored complexes being visible to eye in range of 10-6M to 10-7M . Erochrome ...
Slide 1
... of the ions participate in the reaction. • K+ and NO3- ions are present in solution both before and after the reaction. Ions such as these, which do not participate directly in a reaction in solution, are called spectator ions. • The ions that participate in this reaction are the Ba2+ and CrO42- ion ...
... of the ions participate in the reaction. • K+ and NO3- ions are present in solution both before and after the reaction. Ions such as these, which do not participate directly in a reaction in solution, are called spectator ions. • The ions that participate in this reaction are the Ba2+ and CrO42- ion ...
Molybdenum-Pterin Chemistry. 3. Use of X
... oxidation states has been investigated by X-ray photoelectron spectroscopy (XPS). Prior difficulties in making oxidation state assignments for the metal center and coordinated pterin can be resolved through comparison of Mo 3d binding energies (BE) for these new complexes with the BEs of standard mo ...
... oxidation states has been investigated by X-ray photoelectron spectroscopy (XPS). Prior difficulties in making oxidation state assignments for the metal center and coordinated pterin can be resolved through comparison of Mo 3d binding energies (BE) for these new complexes with the BEs of standard mo ...
Powerpoint
... have no effect on the preference of ion discharge. But some may. • Mercury electrode(汞電極) If graphite or platinum electrodes are used in the electrolysis of concentrated NaCl solution, only H+ is discharged at the cathode. But if mercury electrode is used for the cathode, Na+ is discharged because s ...
... have no effect on the preference of ion discharge. But some may. • Mercury electrode(汞電極) If graphite or platinum electrodes are used in the electrolysis of concentrated NaCl solution, only H+ is discharged at the cathode. But if mercury electrode is used for the cathode, Na+ is discharged because s ...
Mass Spectrometry and Organic
... Take the Weight of ion, divide by 13 This answer is N, for (CH)N and any numerical remainder is added as H e.g.; 92 92/13 = 7 with remainder = 1; C7H8 weighs 92. This is our candidate formula Can evaluate other alternative candidate formulas possessing heteroatoms. For each member of the list below, ...
... Take the Weight of ion, divide by 13 This answer is N, for (CH)N and any numerical remainder is added as H e.g.; 92 92/13 = 7 with remainder = 1; C7H8 weighs 92. This is our candidate formula Can evaluate other alternative candidate formulas possessing heteroatoms. For each member of the list below, ...
Coordination complex

In chemistry, a coordination complex or metal complex consists of a central atom or ion, which is usually metallic and is called the coordination centre, and a surrounding array of bound molecules or ions, that are in turn known as ligands or complexing agents. Many metal-containing compounds, especially those of transition metals, are coordination complexes.