HNRS 227 Lecture #2 Chapters 2 and 3
... masses” ⌧cooler liquids or gasses descend while warmer liquids or gasses rise ...
... masses” ⌧cooler liquids or gasses descend while warmer liquids or gasses rise ...
Chapter 12
... The First Law is a general equation of Conservation of Energy There is no practical, macroscopic, distinction between the results of energy transfer by heat and by work Q and W are related to the properties of state for a system ...
... The First Law is a general equation of Conservation of Energy There is no practical, macroscopic, distinction between the results of energy transfer by heat and by work Q and W are related to the properties of state for a system ...
Chapter 12
... The First Law is a general equation of Conservation of Energy There is no practical, macroscopic, distinction between the results of energy transfer by heat and by work Q and W are related to the properties of state for a system ...
... The First Law is a general equation of Conservation of Energy There is no practical, macroscopic, distinction between the results of energy transfer by heat and by work Q and W are related to the properties of state for a system ...
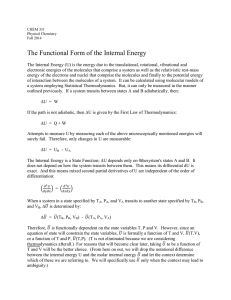
The Functional Form of the Internal Energy
... The Functional Form of the Internal Energy The Internal Energy (U) is the energy due to the translational, rotational, vibrational and electronic energies of the molecules that comprise a system as well as the relativistic rest-mass energy of the electrons and nuclei that comprise the molecules and ...
... The Functional Form of the Internal Energy The Internal Energy (U) is the energy due to the translational, rotational, vibrational and electronic energies of the molecules that comprise a system as well as the relativistic rest-mass energy of the electrons and nuclei that comprise the molecules and ...
Thermodynamics
... Thermodynamic free energy • The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of ene ...
... Thermodynamic free energy • The thermodynamic free energy is the amount of work that a thermodynamic system can perform. The concept is useful in the thermodynamics of chemical or thermal processes in engineering and science. The free energy is the internal energy of a system less the amount of ene ...
Internal Energy, Heat, Enthalpy, and Calorimetry
... What is Enthalpy and How Does it Relate to Internal Energy (E)? If a process takes place at constant pressure (as the majority of processes we study do) and the only work done is this pressure–volume work, we can account for heat flow during the process by measuring the enthalpy of the system E ...
... What is Enthalpy and How Does it Relate to Internal Energy (E)? If a process takes place at constant pressure (as the majority of processes we study do) and the only work done is this pressure–volume work, we can account for heat flow during the process by measuring the enthalpy of the system E ...
Welcome to Thermochemistry!
... to do work and is the sum of its enthalpy (H) plus the product of the temperature and the entropy (S) of the system. This quantity can be defined as: G=H−TS or more completely as G=U+PV−TS where •U = internal energy (SI unit: joule) •P = pressure (SI unit: pascal) •V = volume (SI unit: m 3 ) •T = te ...
... to do work and is the sum of its enthalpy (H) plus the product of the temperature and the entropy (S) of the system. This quantity can be defined as: G=H−TS or more completely as G=U+PV−TS where •U = internal energy (SI unit: joule) •P = pressure (SI unit: pascal) •V = volume (SI unit: m 3 ) •T = te ...
Energy
... – The sum of the kinetic and potential energies of all the “particles” in the system – An increase in the internal energy of a system can take three forms • An increase in temperature • A phase change • The initiation of a chemical reaction – A decrease in the internal energy of a system will usuall ...
... – The sum of the kinetic and potential energies of all the “particles” in the system – An increase in the internal energy of a system can take three forms • An increase in temperature • A phase change • The initiation of a chemical reaction – A decrease in the internal energy of a system will usuall ...
Principles of Technology
... universe. The laws of thermodynamics are based on our experiences in observing nature. Thermodynamics has many applications in disciplines ranging from physics and engineering to biology and medicine. The First Law of Thermodynamics The first law of thermodynamics is a restatement of the law of cons ...
... universe. The laws of thermodynamics are based on our experiences in observing nature. Thermodynamics has many applications in disciplines ranging from physics and engineering to biology and medicine. The First Law of Thermodynamics The first law of thermodynamics is a restatement of the law of cons ...
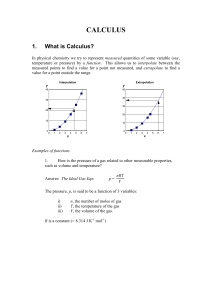
CALCULUS
... e) From (c) above, if rate is R and temperature is T, then the shorthand for (c) is to write R(T). f) If energy is E and frequency is , then the shorthand for (d) is E(). This means that if we know the value of the symbol in brackets, we can calculate the value of the symbol outside the brackets. ...
... e) From (c) above, if rate is R and temperature is T, then the shorthand for (c) is to write R(T). f) If energy is E and frequency is , then the shorthand for (d) is E(). This means that if we know the value of the symbol in brackets, we can calculate the value of the symbol outside the brackets. ...
Heat

In physics, heat is energy in a process of transfer between a system and its surroundings, other than as work or with the transfer of matter. When there is a suitable physical pathway, heat flows from a hotter body to a colder one. The pathway can be direct, as in conduction and radiation, or indirect, as in convective circulation.Because it refers to a process of transfer between two systems, the system of interest, and its surroundings considered as a system, heat is not a state or property of a single system. If heat transfer is slow and continuous, so that the temperature of the system of interest remains well defined, it can sometimes be described by a process function.Kinetic theory explains heat as a macroscopic manifestation of the motions and interactions of microscopic constituents such as molecules and photons.In calorimetry, sensible heat is defined with respect to a specific chosen state variable of the system, such as pressure or volume. Sensible heat transferred into or out of the system under study causes change of temperature while leaving the chosen state variable unchanged. Heat transfer that occurs with the system at constant temperature and that does change that particular state variable is called latent heat with respect to that variable. For infinitesimal changes, the total incremental heat transfer is then the sum of the latent and sensible heat increments. This is a basic paradigm for thermodynamics, and was important in the historical development of the subject.The quantity of energy transferred as heat is a scalar expressed in an energy unit such as the joule (J) (SI), with a sign that is customarily positive when a transfer adds to the energy of a system. It can be measured by calorimetry, or determined by calculations based on other quantities, relying on the first law of thermodynamics.