Multivalent Ionic Compounds
... electrons = # of protons (In a neutral atom) It has 3 electron shells, so it is in period 3 It has 8 electrons in the outer (valence) shell Page 4 of 35 ...
... electrons = # of protons (In a neutral atom) It has 3 electron shells, so it is in period 3 It has 8 electrons in the outer (valence) shell Page 4 of 35 ...
CHM100PracticeExam2
... 18. Which of the following masses has the highest precision? A) 900.075 B) 8400.00 C) 68.0088 D) 0.00004 19. What is the total number of atoms in 2.70 moles of aluminum (NA=6.023 x 1023) A) 1.63 x 1024 B) 1.62 x 1025 C) 6.023 x 1023 D) 1.63 x 10-24 20. How many moles are in 25.8g of sodium? A) 593.1 ...
... 18. Which of the following masses has the highest precision? A) 900.075 B) 8400.00 C) 68.0088 D) 0.00004 19. What is the total number of atoms in 2.70 moles of aluminum (NA=6.023 x 1023) A) 1.63 x 1024 B) 1.62 x 1025 C) 6.023 x 1023 D) 1.63 x 10-24 20. How many moles are in 25.8g of sodium? A) 593.1 ...
42.89 KB
... 18. At very high pressures (~ 1000 atm), the measured pressure exerted by real gases is greater than that predicted by the ideal gas equation. This is mainly because A) the volume occupied by the gas molecules themselves becomes significant. B) real gases will condense to form solids at 1000 atm pre ...
... 18. At very high pressures (~ 1000 atm), the measured pressure exerted by real gases is greater than that predicted by the ideal gas equation. This is mainly because A) the volume occupied by the gas molecules themselves becomes significant. B) real gases will condense to form solids at 1000 atm pre ...
GCSE_C2_Revision_+_Exam_Questions
... Compounds are substances in which atoms of two, or more,el ements are not just mixed together but chemically combined. Chemical bonding involves either transferring or sharing electrons in the highest occupied energy levels (shells) of atoms. When atoms form chemical bonds by transferring electrons, ...
... Compounds are substances in which atoms of two, or more,el ements are not just mixed together but chemically combined. Chemical bonding involves either transferring or sharing electrons in the highest occupied energy levels (shells) of atoms. When atoms form chemical bonds by transferring electrons, ...
Chapter 8 Test Review
... • Sulfur difluoride • Silicon tetrachloride • Chlorine trifluoride • Tetrasulfur heptanitride ...
... • Sulfur difluoride • Silicon tetrachloride • Chlorine trifluoride • Tetrasulfur heptanitride ...
Final Exam Review
... Atoms must give away electrons to reach a stable octet. Atoms share valence electrons only with neighboring atoms to reach a stable octet. Delocalized electrons move among many atoms creating a sea of electrons. ...
... Atoms must give away electrons to reach a stable octet. Atoms share valence electrons only with neighboring atoms to reach a stable octet. Delocalized electrons move among many atoms creating a sea of electrons. ...
Honors-Final-Review-2014
... _____ boiling point _____ surfactant _____ viscosity _____ solution _____ surface tension ...
... _____ boiling point _____ surfactant _____ viscosity _____ solution _____ surface tension ...
Metallic and nonmetallic double perovskites: A case study of A $ _2
... the Re sublattice. Indeed, because there are two t2g electrons per Re, a large enough splitting would lead to an insulating state. The magnetoresistance of these systems shows an interesting behavior. The Ca compound did not show any significant MR even at high fields. Hence, in Fig. 4, we compare ...
... the Re sublattice. Indeed, because there are two t2g electrons per Re, a large enough splitting would lead to an insulating state. The magnetoresistance of these systems shows an interesting behavior. The Ca compound did not show any significant MR even at high fields. Hence, in Fig. 4, we compare ...
Unit 3: Bonding and Nomenclature Content Outline: Chemical
... B. This term is usually used with molecules that are bound together using covalent bonds. C. These molecules can possess single bonds (-), double bonds (=), or even triple bonds (Ξ). 1. The purpose of “creating” the bonds is to achieve the lowest possible Potential Energy state by filling the valenc ...
... B. This term is usually used with molecules that are bound together using covalent bonds. C. These molecules can possess single bonds (-), double bonds (=), or even triple bonds (Ξ). 1. The purpose of “creating” the bonds is to achieve the lowest possible Potential Energy state by filling the valenc ...
File
... C. Energy is added or removed and temperature stays the same D. None of the above 96. What is specific heat? A. a measure of kinetic energy B. the energy of motion C. how much space something takes up D. the amount of energy required to raise the temperature of a substance 97. Which requires the mos ...
... C. Energy is added or removed and temperature stays the same D. None of the above 96. What is specific heat? A. a measure of kinetic energy B. the energy of motion C. how much space something takes up D. the amount of energy required to raise the temperature of a substance 97. Which requires the mos ...
Name: Northwest Vista College Chem 1311
... A) transition metals B) halogens C) alkali metals D) alkaline earth metals E) noble gases 34. Which one of the following elements is a transition element? A) antimony B) barium C) chromium D) potassium E) selenium 35. According to the zeroth law of thermodynamics: a) Energy is neither lost nor gaine ...
... A) transition metals B) halogens C) alkali metals D) alkaline earth metals E) noble gases 34. Which one of the following elements is a transition element? A) antimony B) barium C) chromium D) potassium E) selenium 35. According to the zeroth law of thermodynamics: a) Energy is neither lost nor gaine ...
Page | 1 MATS1101 Chemistry notes semester 2 2012 TOPIC 1
... A mole of a substance is its atomic, molecular or formula mass expressed in grams Amu = atomic mass unit, where 1amu= 1/12 mass of 1 atom of Carbon-12 (Unit also called the Dalton ...
... A mole of a substance is its atomic, molecular or formula mass expressed in grams Amu = atomic mass unit, where 1amu= 1/12 mass of 1 atom of Carbon-12 (Unit also called the Dalton ...
Chemistry
... 9 – 8 Know that chemical reactions can take place at different rates and that reaction rates depend on a variety of factors that influence the frequency of collision of reactant molecules (e.g. shape and surface area of the reacting species, temperature, pressure, the presence or absence of a cataly ...
... 9 – 8 Know that chemical reactions can take place at different rates and that reaction rates depend on a variety of factors that influence the frequency of collision of reactant molecules (e.g. shape and surface area of the reacting species, temperature, pressure, the presence or absence of a cataly ...
MCQ plus answers
... Your answer as recorded on the sheet will be used in the event of any ambiguity. There is only one correct choice for each question. Negative marks will not be awarded for any question. ...
... Your answer as recorded on the sheet will be used in the event of any ambiguity. There is only one correct choice for each question. Negative marks will not be awarded for any question. ...
Chemistry Final Exam Study Guide
... ____ 75. Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson ____ 76. Dalton's atomic theory included which idea? a. All atoms of all elements are the same size. b. Atoms of different elements always combin ...
... ____ 75. Who was the man who lived from 460B.C.–370B.C. and was among the first to suggest the idea of atoms? a. Atomos c. Democritus b. Dalton d. Thomson ____ 76. Dalton's atomic theory included which idea? a. All atoms of all elements are the same size. b. Atoms of different elements always combin ...
Diodes and Transistors HOW Theq Work
... Valence Electrons and the Atom's Core Look at the sodium atom and the chlorine atom in Figure 4-4. There is a special name for an atom's outer subshell or shell if it is not full. It is called the atom's valence shell. "Valence" means the number of bonds the atom forms. For instance, the valence of ...
... Valence Electrons and the Atom's Core Look at the sodium atom and the chlorine atom in Figure 4-4. There is a special name for an atom's outer subshell or shell if it is not full. It is called the atom's valence shell. "Valence" means the number of bonds the atom forms. For instance, the valence of ...
I. Why Atoms Combine - Manchester High School
... Write the names of both elements. Change the final ending to -ide. ...
... Write the names of both elements. Change the final ending to -ide. ...
Final Exam Review
... acid. heat of solution, H, has these units: kcal per mole. Convert cal to kcal and plug in your data.] (Ch. 10) a. –1.78 kcal/mole d. –17. 8 kcal/mole b. –8.9 kcal/mole e. –17,800 kcal/mole c. –9.81 kcal/mole 32. An isotope of krypton, 89Kr, has a half-life of 3.2 minutes. If the original sample wa ...
... acid. heat of solution, H, has these units: kcal per mole. Convert cal to kcal and plug in your data.] (Ch. 10) a. –1.78 kcal/mole d. –17. 8 kcal/mole b. –8.9 kcal/mole e. –17,800 kcal/mole c. –9.81 kcal/mole 32. An isotope of krypton, 89Kr, has a half-life of 3.2 minutes. If the original sample wa ...
Polyatomic Ions (Memorize for Wednesday, January 31
... Polyatomic ions ending in –ate form acids with an –ic ending EX – H2SO4: sulfate ion changes to sulfuric acid Polyatomic ions ending in –ite form acids with an –ous ending EX – HClO2: chlorite ion changes to chlorous acid The number of hydrogen atoms equals the charge on the polyatomic ion EX – H3PO ...
... Polyatomic ions ending in –ate form acids with an –ic ending EX – H2SO4: sulfate ion changes to sulfuric acid Polyatomic ions ending in –ite form acids with an –ous ending EX – HClO2: chlorite ion changes to chlorous acid The number of hydrogen atoms equals the charge on the polyatomic ion EX – H3PO ...
Grades 9-12 Chemistry California Content Standards
... a. atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. c. salt crystals such as N ...
... a. atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. c. salt crystals such as N ...
Chemistry - Gorman Learning Center
... a. atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. c. salt crystals such as N ...
... a. atoms combine to form molecules by sharing electrons to form covalent or metallic bonds, or by exchanging electrons to form ionic bonds. b. chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. c. salt crystals such as N ...
01.CN_Other pages/p1-9
... (b) (i) Which particle(s) is / are the ions? Hint 2 (ii) What is the relationship between P and Q? (iii) Do particles of P and Q have the same chemical properties? Explain your answer. (c) (i) Suggest a term to indicate the relationship between S and T. (ii) Explain why S and T have the same chemica ...
... (b) (i) Which particle(s) is / are the ions? Hint 2 (ii) What is the relationship between P and Q? (iii) Do particles of P and Q have the same chemical properties? Explain your answer. (c) (i) Suggest a term to indicate the relationship between S and T. (ii) Explain why S and T have the same chemica ...
Chapter 4 - Aqueous Reactions
... A metal can be oxidized by any ion below it Metals above H, react with acids to give H2 The further up the series, the more readily the metal is oxidized See your textbook (p124) for more elements ...
... A metal can be oxidized by any ion below it Metals above H, react with acids to give H2 The further up the series, the more readily the metal is oxidized See your textbook (p124) for more elements ...
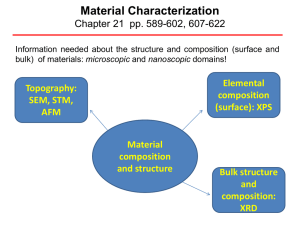
Material Characterization
... IBM Zürich), the Nobel Prize in Physics in 1986. For an STM, good resolution is considered to be 0.1 nm lateral resolution and 0.01 nm depth resolution. With this resolution, individual atoms within materials are routinely imaged and manipulated. The STM can be used not only in ultra high vacuum but ...
... IBM Zürich), the Nobel Prize in Physics in 1986. For an STM, good resolution is considered to be 0.1 nm lateral resolution and 0.01 nm depth resolution. With this resolution, individual atoms within materials are routinely imaged and manipulated. The STM can be used not only in ultra high vacuum but ...