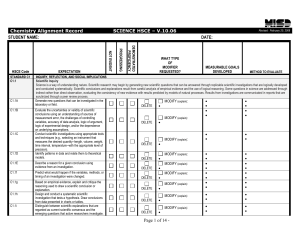
Supplementary Exercise 1B Topic 5
... difference in the tendency to form ions between metal Y and metal W is greater than that between metal X and metal W. Hence metal Y forms ions most readily. For the third chemical cell, the voltmeter gives a negative voltage. Therefore metal W is the negative electrode while metal Z is the positive ...
... difference in the tendency to form ions between metal Y and metal W is greater than that between metal X and metal W. Hence metal Y forms ions most readily. For the third chemical cell, the voltmeter gives a negative voltage. Therefore metal W is the negative electrode while metal Z is the positive ...
Review of N and Metal co-Doped TiO for Water Purification under
... photocatalysts demonstrated more excellent photocatalytic activities for decomposition of enormous organic pollutants in wastewater than doping with N or single metal in the visible light region, further confirming that this combinative method is a promising way of improving the activity. Fig. 1 con ...
... photocatalysts demonstrated more excellent photocatalytic activities for decomposition of enormous organic pollutants in wastewater than doping with N or single metal in the visible light region, further confirming that this combinative method is a promising way of improving the activity. Fig. 1 con ...
Devillez (ld2653) – Test 1 Review – Devillez – (99998)
... In the early 1800s when Dalton formulated his atomic theory, it was believed that all atoms of the same element were the same. The fundamental particles of the atom – protons, neutrons, and electrons – were not known at this time. 034 10.0 points The following are all proposals of Dalton’s atomic th ...
... In the early 1800s when Dalton formulated his atomic theory, it was believed that all atoms of the same element were the same. The fundamental particles of the atom – protons, neutrons, and electrons – were not known at this time. 034 10.0 points The following are all proposals of Dalton’s atomic th ...
THE ADSORPTION OF CO, N2 AND Li ON Ru(109) AND Ru(001
... compared to the terraces of the Ru(109) surface. Nitrogen desorbs from Ru(109) in three distinct desorption processes. A high temperature desorption feature has been assigned to the molecular desorption from the atomic step sites. The electron stimulated dissociation of chemisorbed nitrogen results ...
... compared to the terraces of the Ru(109) surface. Nitrogen desorbs from Ru(109) in three distinct desorption processes. A high temperature desorption feature has been assigned to the molecular desorption from the atomic step sites. The electron stimulated dissociation of chemisorbed nitrogen results ...
Synthesis and Structural Studies of Calcium and Magnesium
... This Dissertation is brought to you for free and open access by the College of Arts and Sciences at SURFACE. It has been accepted for inclusion in Chemistry - Dissertations by an authorized administrator of SURFACE. For more information, please contact surface@syr.edu. ...
... This Dissertation is brought to you for free and open access by the College of Arts and Sciences at SURFACE. It has been accepted for inclusion in Chemistry - Dissertations by an authorized administrator of SURFACE. For more information, please contact surface@syr.edu. ...
PC_Chemistry_Macomb_April08
... Solids can be classified as metallic, ionic, covalent, or network covalent. These different types of solids have different properties that depend on the particles and forces found in the solid. Compare the relative strengths of forces between ...
... Solids can be classified as metallic, ionic, covalent, or network covalent. These different types of solids have different properties that depend on the particles and forces found in the solid. Compare the relative strengths of forces between ...
File
... 36. Hydrogen reacts with some elements to form binary compounds called __________. (Halides, Hydrides, Oxides, all of these) 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Inter ...
... 36. Hydrogen reacts with some elements to form binary compounds called __________. (Halides, Hydrides, Oxides, all of these) 37. The hydrides formed by the transfer of electrons from electropositive metals to hydrogen are called __________. (Ionic hydrides, covalent hydrides, Complex hydrides, Inter ...
Rubidium
... zinnwaldite, which contains traces of up to 1% of its oxide. Lepidolite contains 1.5% rubidium and this is the commercial source of the element. Some potassium minerals and potassium chlorides also contain the element in commercially significant amounts. One notable source is also in the extensive d ...
... zinnwaldite, which contains traces of up to 1% of its oxide. Lepidolite contains 1.5% rubidium and this is the commercial source of the element. Some potassium minerals and potassium chlorides also contain the element in commercially significant amounts. One notable source is also in the extensive d ...
- Kendriya Vidyalaya Jamuna Colliery
... (a) Ge is group 14 elements and In is group 13 element. Therefore, an electron deficit hole is created. Thus semi-conductor is p-type. (b) Since B is a group 13 element and Si is group 14 element, there will be a free electron, thus it is n-type semi-conductor. 5. In terms of band theory what is the ...
... (a) Ge is group 14 elements and In is group 13 element. Therefore, an electron deficit hole is created. Thus semi-conductor is p-type. (b) Since B is a group 13 element and Si is group 14 element, there will be a free electron, thus it is n-type semi-conductor. 5. In terms of band theory what is the ...
enjoy chemistry
... The elements present in Group 18 have their valence shell orbitals completely filled and, therefore, react with a few elements only under certain conditions. Therefore, they are now known as noble gases. (ii)Noble gases are mostly chemically inert. Their inertness to chemical reactivity is attribute ...
... The elements present in Group 18 have their valence shell orbitals completely filled and, therefore, react with a few elements only under certain conditions. Therefore, they are now known as noble gases. (ii)Noble gases are mostly chemically inert. Their inertness to chemical reactivity is attribute ...
Specification – AS/A Level Chemistry A
... These specifications have been developed for students who wish to continue with a study of chemistry at Level 3 in the National Qualifications Framework (NQF). The AS specification has been written to provide progression from GCSE Science and GCSE Additional Science, or from GCSE Chemistry; achievem ...
... These specifications have been developed for students who wish to continue with a study of chemistry at Level 3 in the National Qualifications Framework (NQF). The AS specification has been written to provide progression from GCSE Science and GCSE Additional Science, or from GCSE Chemistry; achievem ...
GCSE Chemistry Specification Specification for exams from 2014 2014
... AQA retains the copyright on all its publications, including the specifications. However, registered centres for AQA are permitted to copy material from this specification booklet for their own internal use, with the following important exception: AQA cannot give permission to centres to photocopy a ...
... AQA retains the copyright on all its publications, including the specifications. However, registered centres for AQA are permitted to copy material from this specification booklet for their own internal use, with the following important exception: AQA cannot give permission to centres to photocopy a ...
Chapter 5: Gases - HCC Learning Web
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
Instructor`s Guide to General Chemistry: Guided
... of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be found. Molecules/ions react and molecules/ions are produced, so the units to keep tr ...
... of the reactants to the number of molecules/ions that are produced as products. The number of molecules/ions is measured in units of moles. (b) Steps 2 and 3 make clear what information is given and what needs to be found. Molecules/ions react and molecules/ions are produced, so the units to keep tr ...
Homework 5-7 answers
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
Homework 5-8 answers
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
... A) the energy stored within the structural units of chemical substances. B) the energy associated with the random motion of atoms and molecules. C) solar energy, i.e. energy that comes from the sun. D) energy available by virtue of an object's position. Ans: C Category: Easy Section: 6.1 2. Thermal ...
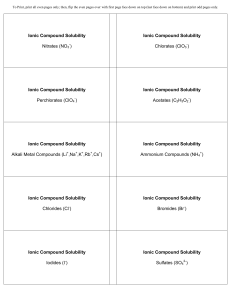
Ionic Compound Solubility Nitrates (NO3 ) Ionic Compound
... Oxygen and a metallic element. Ex: Fe2O3 Oxygen and a nonmentallic element. ...
... Oxygen and a metallic element. Ex: Fe2O3 Oxygen and a nonmentallic element. ...
Solubility and complexes
... Stability constant and stepwise stability constant Acidity of some metal ions in solution Coordination compounds and geometry Nomenclature of coordination compounds Isomerism in Complexes ...
... Stability constant and stepwise stability constant Acidity of some metal ions in solution Coordination compounds and geometry Nomenclature of coordination compounds Isomerism in Complexes ...
BRIEF ANSWERS TO SELECTED PROBLEMS APPENDIX G
... is the volume of the container. Solids and liquids have a definite volume. The volume of the container does not affect the volume of a solid or liquid. (a) gas (b) liquid (c) liquid 1.4 Physical property: a characteristic shown by a substance itself, without any interaction with or change into other ...
... is the volume of the container. Solids and liquids have a definite volume. The volume of the container does not affect the volume of a solid or liquid. (a) gas (b) liquid (c) liquid 1.4 Physical property: a characteristic shown by a substance itself, without any interaction with or change into other ...
STUDY MATERIAL 2016-17 CHEMISTRY CLASS XII
... flow of electrons. Hence conductivity decreases. In case of semi-conductors, with increase of temperature, more electrons can shift from valence band to conduction band. Hence conductivity increases. 8. What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic, why? ...
... flow of electrons. Hence conductivity decreases. In case of semi-conductors, with increase of temperature, more electrons can shift from valence band to conduction band. Hence conductivity increases. 8. What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic, why? ...
Exam Review
... low boiling points are due to the fact that small molecules have fewer electrons and weaker London dispersion forces, compared with large molecules. The fractions with higher boiling points contain much larger molecules. The physical process of fractionation is followed by chemical processes in whic ...
... low boiling points are due to the fact that small molecules have fewer electrons and weaker London dispersion forces, compared with large molecules. The fractions with higher boiling points contain much larger molecules. The physical process of fractionation is followed by chemical processes in whic ...
EIT Review S2012 Part 2 Dr. J. Mack CSUS Department of Chemistry
... A 50.0 g sample of a mixture of MgCO3 and NaCl was heated and the CO2 (g) collected in a 10.0 L flask had a pressure of 755 torr at 20.0° C. How much NaCl was in the original sample? Solution: Recognize that the CO2 (g) produced is related to MgCO3 (s) by: ...
... A 50.0 g sample of a mixture of MgCO3 and NaCl was heated and the CO2 (g) collected in a 10.0 L flask had a pressure of 755 torr at 20.0° C. How much NaCl was in the original sample? Solution: Recognize that the CO2 (g) produced is related to MgCO3 (s) by: ...