CHEM110P1_06_2015_Y_P1
... A student used a titration to determine whether an unknown sample was malonic acid (CH2(COOH)2, molar mass = 104.1 g mol–1). The student weighed 1.08 g of the unknown acid and transferred it to a 250.0 mL volumetric flask and prepared a standard solution. The burette was filled with 0.09970 M NaOH s ...
... A student used a titration to determine whether an unknown sample was malonic acid (CH2(COOH)2, molar mass = 104.1 g mol–1). The student weighed 1.08 g of the unknown acid and transferred it to a 250.0 mL volumetric flask and prepared a standard solution. The burette was filled with 0.09970 M NaOH s ...
Welcome`to`AP`Chemistry!
... Converting)numbers)to)scientific)notation)makes)large)and)small)numbers)easy)to)work)with.))[It)is)even)possible)to)write) numbers)which)are)greater)than)the)total)of)all)of)the)atoms)in)the)universe,)such)as)109283748.]))The)major)advantage,) however,)is)that)calculations)are)simplified)when)scient ...
... Converting)numbers)to)scientific)notation)makes)large)and)small)numbers)easy)to)work)with.))[It)is)even)possible)to)write) numbers)which)are)greater)than)the)total)of)all)of)the)atoms)in)the)universe,)such)as)109283748.]))The)major)advantage,) however,)is)that)calculations)are)simplified)when)scient ...
Chemistry Entrance Material for Grade 11 to 12
... Based on this data alone, which of the above substances is expected to have the highest molar heat of fusion? 31. The temperature at the boiling point or melting point stays the same). ...
... Based on this data alone, which of the above substances is expected to have the highest molar heat of fusion? 31. The temperature at the boiling point or melting point stays the same). ...
Reactions of Plutonium Dioxide with Water and Oxygen
... with an oxygen-hydrogen mixture show that the oxide is both chemically reactive and catalytically active. Water dissociatively adsorbs on the oxide below 10(YC and dcsorbs at higher temperatures, while reacting at a relatively slow rate to form a higher oxide, PU02+X,and H2. Exposure of a 21, mixtur ...
... with an oxygen-hydrogen mixture show that the oxide is both chemically reactive and catalytically active. Water dissociatively adsorbs on the oxide below 10(YC and dcsorbs at higher temperatures, while reacting at a relatively slow rate to form a higher oxide, PU02+X,and H2. Exposure of a 21, mixtur ...
Chemical reaction
... This question can be answered very easily based on the mole:mole ratios that are inherent in a balanced chemical equation. For example for the above equation 2 moles of C4H10 will react with every 13 moles of O2. This can also be stated as an equality: 2 mole C4H10 = 13 mole O2, this equality can be ...
... This question can be answered very easily based on the mole:mole ratios that are inherent in a balanced chemical equation. For example for the above equation 2 moles of C4H10 will react with every 13 moles of O2. This can also be stated as an equality: 2 mole C4H10 = 13 mole O2, this equality can be ...
Chem 11 Stoichiometry (mol-mol) Using the formulas we have
... Using the coefficients as moles, we can determine the mass of the each of the reactants present and also for the product formed. Mass of Reactants: m = nxM = (1 mol N2)(28.0134g/mol N2) = 28.0134g N2 m = nxM = (3 mol H2)(2.01588g/mol H2) = 6.04764g H2 Total mass of Reactants = 28.0134g N2 + 6.04764g ...
... Using the coefficients as moles, we can determine the mass of the each of the reactants present and also for the product formed. Mass of Reactants: m = nxM = (1 mol N2)(28.0134g/mol N2) = 28.0134g N2 m = nxM = (3 mol H2)(2.01588g/mol H2) = 6.04764g H2 Total mass of Reactants = 28.0134g N2 + 6.04764g ...
Chapter 14
... Third law of thermodynamics: The absolute entropy (S) of a perfect crystal of any pure substance at absolute zero is 0.0 J/mol.K. Because there are standard ways of find the change in entropy for a pure substance as we change the temperature of the substance at constant pressure, the third law of t ...
... Third law of thermodynamics: The absolute entropy (S) of a perfect crystal of any pure substance at absolute zero is 0.0 J/mol.K. Because there are standard ways of find the change in entropy for a pure substance as we change the temperature of the substance at constant pressure, the third law of t ...
Chemical Reactions
... The equation we have written is incomplete, however. While it tells us the formulas of the starting materials and products (which every chemical equation must do) and the physical state of each reactant and product, it does not give the amounts correctly. It is not balanced, which means that the num ...
... The equation we have written is incomplete, however. While it tells us the formulas of the starting materials and products (which every chemical equation must do) and the physical state of each reactant and product, it does not give the amounts correctly. It is not balanced, which means that the num ...
CHAPTER 4: AQUEOUS REACTIONS AND SOLUTION
... following compounds is dissolved in 1L of water: Ca(NO3)2, C6H12O6 (glucose), CH3COONa (sodium acetate), and CH3COOH (acetic acid). Rank the solutions in order of increasing electrical conductivity based on the fact that the greater the number of ions in solution the greater the conductivity. ...
... following compounds is dissolved in 1L of water: Ca(NO3)2, C6H12O6 (glucose), CH3COONa (sodium acetate), and CH3COOH (acetic acid). Rank the solutions in order of increasing electrical conductivity based on the fact that the greater the number of ions in solution the greater the conductivity. ...
AP® Chemistry
... Directions: Each set of lettered choices below refers to the numbered statements immediately following it. Select the one that is best in each case and then place the letter of your choice in the corresponding box on the student answer sheet. A choice may be used once, more than once, or not at all ...
... Directions: Each set of lettered choices below refers to the numbered statements immediately following it. Select the one that is best in each case and then place the letter of your choice in the corresponding box on the student answer sheet. A choice may be used once, more than once, or not at all ...
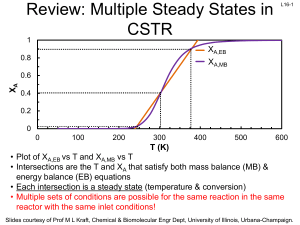
Review: SS Nonisothermal Reactors - University of Illinois Urbana
... The reactor is well-insulated, so no heat is lost to the surroundings. To control the temperature, an inert liquid C is added to the reaction. The flow rate of C is adjusted to keep T constant at 100 °F. What is the flow rate of C after 2h? TC0 = 80 °F V0= 50 ft3 H°RX=-25000 Btu/lb mol k(100 °F)= 1 ...
... The reactor is well-insulated, so no heat is lost to the surroundings. To control the temperature, an inert liquid C is added to the reaction. The flow rate of C is adjusted to keep T constant at 100 °F. What is the flow rate of C after 2h? TC0 = 80 °F V0= 50 ft3 H°RX=-25000 Btu/lb mol k(100 °F)= 1 ...
Chemistry Standards Clarification
... Academy, in collaboration with the Michigan Mathematics and Science Center Network and the Michigan Science Teachers Association, worked in partnership with Michigan Department of Education to develop this companion document. Our goal is for each student to master the science content expectations as ...
... Academy, in collaboration with the Michigan Mathematics and Science Center Network and the Michigan Science Teachers Association, worked in partnership with Michigan Department of Education to develop this companion document. Our goal is for each student to master the science content expectations as ...
Asymmetry of Electron Transmission through Monolayers of Helical
... electron-transfer direction is from the C-terminal end to the N-terminal end (Figure 5). The electron-transfer rates through polyalanine derivative monolayer are increased for negative overpotentials; thus the rate constant kc is higher than ka at given absolute value of overpotential. The average r ...
... electron-transfer direction is from the C-terminal end to the N-terminal end (Figure 5). The electron-transfer rates through polyalanine derivative monolayer are increased for negative overpotentials; thus the rate constant kc is higher than ka at given absolute value of overpotential. The average r ...
Worked out problems
... are balanced by adding seven H2O to the products. The 14 hydrogen atoms in 7 H2O are then balanced by adding 14 H+ to the reactants: We then balance the charge by adding electrons to the left side of the equation so that the total charge is the same on the two sides: We can check this result by look ...
... are balanced by adding seven H2O to the products. The 14 hydrogen atoms in 7 H2O are then balanced by adding 14 H+ to the reactants: We then balance the charge by adding electrons to the left side of the equation so that the total charge is the same on the two sides: We can check this result by look ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.