Chapter 18 - Sarah Mahajan Study Guides
... o Catalysts remain unchanged and don’t affect the amount of reactants at products, only the rate that it takes them to achieve equilibrium o Inhibitors interfere with catalysts ...
... o Catalysts remain unchanged and don’t affect the amount of reactants at products, only the rate that it takes them to achieve equilibrium o Inhibitors interfere with catalysts ...
Chapter 14, Section 1, pages 494-501
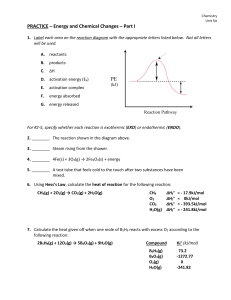
... A chemical equilibrium is a state of balance between the forward and reverse reactions. The concentration of products and reactants remains unchanged. H2 + I2 <--------------> 2HI See fig. 3, pg. 498 Chemical Equilibria Are Dynamic (Leaky boat) Demo-pg. 500 Static equilibrium is a state when nothing ...
... A chemical equilibrium is a state of balance between the forward and reverse reactions. The concentration of products and reactants remains unchanged. H2 + I2 <--------------> 2HI See fig. 3, pg. 498 Chemical Equilibria Are Dynamic (Leaky boat) Demo-pg. 500 Static equilibrium is a state when nothing ...
2.4 Chemical Reactions and Enzymes
... Chemical reactions that release energy often occur on their own, or spontaneously. ...
... Chemical reactions that release energy often occur on their own, or spontaneously. ...
KEY CONCEPT Enzymes are catalysts for chemical
... – Enzymes are needed for almost all processes. – Most enzymes are proteins. ...
... – Enzymes are needed for almost all processes. – Most enzymes are proteins. ...
Title - Iowa State University
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
... 3. Which of the following statements about catalysts is false? a. A catalyst will speed up the rate of a reaction. b. Catalysts are used in very many commercially important chemical reactions. c. Catalytic converters are examples of heterogeneous catalysts. d. A catalyst can cause a nonspontaneous r ...
Lecture 8
... ΔS and ΔG for reactions do not depend on the reaction pathway. They depend even more strongly than ΔH on concentration and pressure. ...
... ΔS and ΔG for reactions do not depend on the reaction pathway. They depend even more strongly than ΔH on concentration and pressure. ...
Ch 5.1 The Nature of Chemical Reactions
... • Understand parts to a chemical equation (reactants, products, yeild sign, double arrow) • Conservation of matter is expressed through balancing chemical equations • Describe difference between endothermic and exothermic reactions ...
... • Understand parts to a chemical equation (reactants, products, yeild sign, double arrow) • Conservation of matter is expressed through balancing chemical equations • Describe difference between endothermic and exothermic reactions ...
1 ChE 505 WORKSHOP 1 1. Why are chemical reactions important
... Can the temperature and composition dependence term be separated in reaction rates that follow the Languir-Hinshelwood form? Can an activation energy be defined? ...
... Can the temperature and composition dependence term be separated in reaction rates that follow the Languir-Hinshelwood form? Can an activation energy be defined? ...
AP Chemistry
... 1. Ionic and molecular species present in chemical systems: net ionic equations 2. Balancing of equations, including those for redox reactions 3. Mass and volume relations with emphasis on the mole concept, including empirical formulas and limiting reactants C. Equilibrium 1. Concept of dynamic equi ...
... 1. Ionic and molecular species present in chemical systems: net ionic equations 2. Balancing of equations, including those for redox reactions 3. Mass and volume relations with emphasis on the mole concept, including empirical formulas and limiting reactants C. Equilibrium 1. Concept of dynamic equi ...
Syllabus
... Part3: An Introduction to using Spartan for Quantum Mechanical Calculations. Building molecules, determination of physical properties, examining the energetics of reactions Part 4: Important Formulae from Statistical Thermodynamics: Partition functions for electronic. translational, rotational and v ...
... Part3: An Introduction to using Spartan for Quantum Mechanical Calculations. Building molecules, determination of physical properties, examining the energetics of reactions Part 4: Important Formulae from Statistical Thermodynamics: Partition functions for electronic. translational, rotational and v ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.