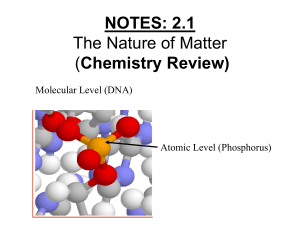
Name: Date: AP Chemistry/Chemistry 145 Summer Assignment
... 19. A 11.6-g sample of iron ore, containing Fe3O4 (232 g/mol), is reacted with carbon to form purified iron. The other product of the reaction is carbon dioxide gas. 2.10 g of iron is recovered from one such trial. ...
... 19. A 11.6-g sample of iron ore, containing Fe3O4 (232 g/mol), is reacted with carbon to form purified iron. The other product of the reaction is carbon dioxide gas. 2.10 g of iron is recovered from one such trial. ...
I, I, I, 4- Measurement Unit Conversions- Kilo
... Describe trends in properties (e.g., ionization energy or reactivity as a function of location on the periodic table, boiling point of organic liquids as a function of molecular weight). Atomic radius is one-half of the distance between the center of identical atoms that are not bonded together. Ion ...
... Describe trends in properties (e.g., ionization energy or reactivity as a function of location on the periodic table, boiling point of organic liquids as a function of molecular weight). Atomic radius is one-half of the distance between the center of identical atoms that are not bonded together. Ion ...
Acc
... Count atoms on both sides (polyatomics can be counted as one if they are on both sides) Balance using coefficients (save H & O for last if they’re present) Check balancing and make sure coefficients are in the lowest ratio ...
... Count atoms on both sides (polyatomics can be counted as one if they are on both sides) Balance using coefficients (save H & O for last if they’re present) Check balancing and make sure coefficients are in the lowest ratio ...
Chemistry
... a. aqueous barium chloride reacts with aqueous silver sulfate to form solid silver chloride and solid barium sulfate b. calcium carbonate when heated forms calcium oxide and carbon dioxide ...
... a. aqueous barium chloride reacts with aqueous silver sulfate to form solid silver chloride and solid barium sulfate b. calcium carbonate when heated forms calcium oxide and carbon dioxide ...
Ch. 10 – Stoichiometry Stoichiometry – relates molar ratios between
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
... These molar ratios are used to 'convert' between any two compounds, whether they are reactants or products. This allows us to calculate moles of reactants needed, or products produced. ...
Enzyme Activity
... • Active site: The region of an enzyme molecule which binds the substrate and carries out the catalytic reaction • Enzyme : A biological catalyst. Usually a globular protein molecule produced by living organisms that can speed up a specific chemical reaction without itself being destroyed or changed ...
... • Active site: The region of an enzyme molecule which binds the substrate and carries out the catalytic reaction • Enzyme : A biological catalyst. Usually a globular protein molecule produced by living organisms that can speed up a specific chemical reaction without itself being destroyed or changed ...
45. kinetics ch 12
... quantity that expresses how the concentration of a reactant or product changes with time. The rates of reactions span an enormous range. From those that are complete within seconds to those that take thousands or even millions of years. ...
... quantity that expresses how the concentration of a reactant or product changes with time. The rates of reactions span an enormous range. From those that are complete within seconds to those that take thousands or even millions of years. ...
Chemical Equations and Reaction Types Lab
... The purpose of this laboratory exercise is to develop skills in writing and balancing chemical equations. The relevance of this exercise is illustrated by a series of demonstration reactions, performed by your lab instructor, which will provide a model to enable the student to predict the products a ...
... The purpose of this laboratory exercise is to develop skills in writing and balancing chemical equations. The relevance of this exercise is illustrated by a series of demonstration reactions, performed by your lab instructor, which will provide a model to enable the student to predict the products a ...
Chemical reactions
... • Occur through formation and breaking of chemical bonds between atoms • Involve changes in matter, creation of new materials and energy exchange • Chemical equations - concise representation of chemical reactions ...
... • Occur through formation and breaking of chemical bonds between atoms • Involve changes in matter, creation of new materials and energy exchange • Chemical equations - concise representation of chemical reactions ...
+ NO 2
... CHEMICAL REACTION (BONDS MUST BREAK) • SURFACE AREA/ CONTACT AREA (OPPORTUNITY FOR COLLISIONS) • CONCENTRATION ( INCREASE FREQUENCY) • TEMPERATURE ( INCREASE FREQUENCY) • CATALYST ( EFFECTIVE COLLISIONS) • NATURE OF REACTANTS ( EFFECTIVE COLLISIONS) ...
... CHEMICAL REACTION (BONDS MUST BREAK) • SURFACE AREA/ CONTACT AREA (OPPORTUNITY FOR COLLISIONS) • CONCENTRATION ( INCREASE FREQUENCY) • TEMPERATURE ( INCREASE FREQUENCY) • CATALYST ( EFFECTIVE COLLISIONS) • NATURE OF REACTANTS ( EFFECTIVE COLLISIONS) ...
Physical Chemistry
... The book is divided into four parts. The first part focuses on the macroscopic properties of physical systems. It begins with the descriptive study of gases and liquids, and proceeds to the study of thermodynamics, which is a comprehensive macroscopic theory of the behavior of material systems. The ...
... The book is divided into four parts. The first part focuses on the macroscopic properties of physical systems. It begins with the descriptive study of gases and liquids, and proceeds to the study of thermodynamics, which is a comprehensive macroscopic theory of the behavior of material systems. The ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.