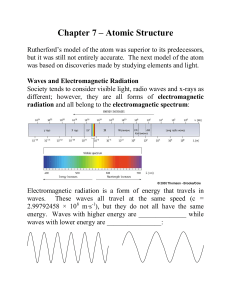
Press Release Equivalence principle also valid for atoms
... Equivalence principle also valid for atoms Garching and Tübingen physicists have on an “atomic fountain” carried out Galilei’s leaning tower experiment with quantum objects In the 16th century Galileo Galilei dropped leaden, golden and wooden objects off the leaning tower in Pisa. He found that all ...
... Equivalence principle also valid for atoms Garching and Tübingen physicists have on an “atomic fountain” carried out Galilei’s leaning tower experiment with quantum objects In the 16th century Galileo Galilei dropped leaden, golden and wooden objects off the leaning tower in Pisa. He found that all ...
Document
... electron orbital. • Sublevels (l) describe the shape of orbitals. The number of sublevels = n • The direction (m) describes orientation of the sublevels. • Spin (s) refers to how an electron spins. ...
... electron orbital. • Sublevels (l) describe the shape of orbitals. The number of sublevels = n • The direction (m) describes orientation of the sublevels. • Spin (s) refers to how an electron spins. ...
The Kronig-Penney Model Motivation Andrew D. Baczewski October 31, 2011
... In this form, for a fixed value of P , it is rather simple to plot the left hand side as a function of Ka. When the value of the left hand side of this Equation is between -1 and 1 (the range of cos(ka) for real arguments), there will be a value of k for which a solution exists. When the value is o ...
... In this form, for a fixed value of P , it is rather simple to plot the left hand side as a function of Ka. When the value of the left hand side of this Equation is between -1 and 1 (the range of cos(ka) for real arguments), there will be a value of k for which a solution exists. When the value is o ...
Atom and Light
... – A cool, transparent gas in front of a source of a continuous spectrum produces an absorption line spectrum. The absorption lines in the absorption line spectrum of a particular gas occur at exactly the same wavelengths as the emission lines in the emission line spectrum of the same gas. ...
... – A cool, transparent gas in front of a source of a continuous spectrum produces an absorption line spectrum. The absorption lines in the absorption line spectrum of a particular gas occur at exactly the same wavelengths as the emission lines in the emission line spectrum of the same gas. ...
Slide 1
... The resulting expressions that need to be evaluated numerically contain very large number of terms, resulting in tedious coding and debugging. The symbolic program generator was developed for this purpose to automatically generate efficient numerical codes for coupled-cluster or perturbation theory ...
... The resulting expressions that need to be evaluated numerically contain very large number of terms, resulting in tedious coding and debugging. The symbolic program generator was developed for this purpose to automatically generate efficient numerical codes for coupled-cluster or perturbation theory ...
Detailed Notes CH. 6
... A collection of orbitals with the same value of n is called an electron shell. • There are n2 orbitals in a shell described by a the n value. • For example, for n = 3, there are 32 = 9 orbitals. • A set of orbitals with the same n and l is called a subshell. • Each subshell is designated by a number ...
... A collection of orbitals with the same value of n is called an electron shell. • There are n2 orbitals in a shell described by a the n value. • For example, for n = 3, there are 32 = 9 orbitals. • A set of orbitals with the same n and l is called a subshell. • Each subshell is designated by a number ...
LEP 5.1.08 Atomic spectra of two-electron systems: He, Hg
... unit are used as a source of radiation. The power supply is adjusted to about 5 kV. The scale is attached directly behind the spectral tube in order to minimize parallax errors. The diffraction grating should be set up at about 50 cm and at the same height as the spectral tube. The grating must be a ...
... unit are used as a source of radiation. The power supply is adjusted to about 5 kV. The scale is attached directly behind the spectral tube in order to minimize parallax errors. The diffraction grating should be set up at about 50 cm and at the same height as the spectral tube. The grating must be a ...
On the Sympathetic Cooling of Atomic and Molecular Ions with
... spatially with a small cloud of 40Ca+ or 23Na+ ions trapped in a co-located linear Paul radiofrequency trap, which we have constructed. We have indications that both traps can operate simultaneously without interference. We have successfully observed Na MOTs on two transitions (3s F=2->3p F’=3 and F ...
... spatially with a small cloud of 40Ca+ or 23Na+ ions trapped in a co-located linear Paul radiofrequency trap, which we have constructed. We have indications that both traps can operate simultaneously without interference. We have successfully observed Na MOTs on two transitions (3s F=2->3p F’=3 and F ...
Bourdel-3 (doc, 273 KiB)
... Atomic gases have proven to be a useful ressource for precision measurements of the atom properties or of the external forces acting on them. For example, atom interferometers permit the measurement of the local gravity constant g with a relative accuracy of the order of 10 -8 at 1s 1. A long observ ...
... Atomic gases have proven to be a useful ressource for precision measurements of the atom properties or of the external forces acting on them. For example, atom interferometers permit the measurement of the local gravity constant g with a relative accuracy of the order of 10 -8 at 1s 1. A long observ ...
Nature template - PC Word 97
... Atomic gases have proven to be a useful ressource for precision measurements of the atom properties or of the external forces acting on them. For example, atom interferometers permit the measurement of the local gravity constant g with a relative accuracy of the order of 10 -8 at 1s 1. A long observ ...
... Atomic gases have proven to be a useful ressource for precision measurements of the atom properties or of the external forces acting on them. For example, atom interferometers permit the measurement of the local gravity constant g with a relative accuracy of the order of 10 -8 at 1s 1. A long observ ...
orbital
... for an electron with a given energy, the best we can do is describe a region in the atom of high probability of finding it – called an orbital ◦ a probability distribution map of a region where the electron is likely to be found ◦ distance vs. y2 (wave function) many of the properties of atoms are r ...
... for an electron with a given energy, the best we can do is describe a region in the atom of high probability of finding it – called an orbital ◦ a probability distribution map of a region where the electron is likely to be found ◦ distance vs. y2 (wave function) many of the properties of atoms are r ...